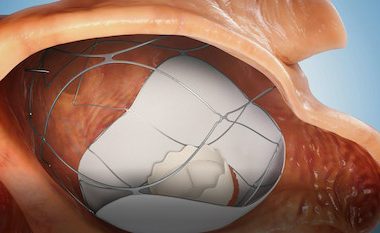
CAESAREA, Israel, Feb. 1, 2023 /PRNewswire/ — AllMeD Solutions (TASE: ALMD) announced subsidiary TruLeaf Medical’s receipt of the Helsinki Ethics Committee’s approval to conduct clinical trial in human subjects As part of the trial, a prosthetic mitral valve will be implanted via two needle sticks in the groins in a two-stage catheterization procedure without the need for […]
Author: Ken Dropiewski
AMGEN REPORTS FOURTH QUARTER AND FULL YEAR 2022 FINANCIAL RESULTS
AMGEN ALSO PROVIDES 2023 GUIDANCE EXCLUDING ANY CONTRIBUTION FROM THE ANNOUNCED ACQUISITION OF HORIZON THERAPEUTICS THOUSAND OAKS, Calif., Jan. 31, 2023 /PRNewswire/ — Amgen (NASDAQ: AMGN) today announced financial results for the fourth quarter and full year 2022 versus comparable periods in 2021. “We executed effectively in 2022, delivering strong volume growth, advancing numerous […]
BioCardia and CellProthera Enhance Collaboration for Development of ProtheraCytes™ for the Treatment of Acute Myocardial Infarction in Europe and Potential Early Access for Patients
SUNNYVALE, Calif. and MULHOUSE, France, Feb. 01, 2023 (GLOBE NEWSWIRE) — BioCardia, Inc. [Nasdaq: BCDA], a developer of cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary diseases, and CellProthera, a private developer of cell-based therapies to repair ischemic tissues, today announce an amendment to their Clinical Research Supply and Support […]
Sequana Medical announces grant of additional DSR® patent in United States
Ghent, Belgium – 1 February 2023 – Sequana Medical NV (Euronext Brussels: SEQUA) (the “Company” or “Sequana Medical“), a pioneer in the treatment of fluid overload in liver disease, heart failure and cancer, today announces the grant of an additional US patent for its DSR (Direct Sodium Removal) program. US patent number 11,559,618 B2 has been granted on 24 […]
CIS Launches National Cardiology Platform in Partnership With Lee Equity Partners
HOUMA, La.–(BUSINESS WIRE)–Cardiovascular Institute of the South (CIS), one of the largest independent cardiovascular practices in the country, announced the launch of a national cardiovascular platform, Cardiovascular Logistics, in partnership with private equity firm Lee Equity Partners, headquartered in New York City. The launch of Cardiovascular Logistics will build and […]
Bayer Partners with Huma on Bayer® Aspirin Heart Risk Assessment Online Educational Tool to Raise Awareness of Heart Health and its Risk Factors
Huma’s predictive algorithm will power a new global online patient engagement tool* that provides an accessible solution for heart health education NEW YORK, Feb. 1, 2023 /PRNewswire/ — Huma Therapeutics Limited (“Huma”), a leading global digital health company, and Bayer, a leading global life sciences company, have partnered to develop the Bayer Aspirin […]
Occlutech Continues its Progress in US FDA Study OCCLUFLEX – Enrolls First Patient in Europe
SCHAFFHAUSEN, CH / ACCESSWIRE / January 31, 2023 / Occlutech Holding AG Occlutech Holding AG (“Occlutech”), one of the world’s leading providers of minimally invasive structural heart disease devices, continues its progress in the US market. The first patient has now been enrolled in the global US study OCCLUFLEX in […]
CytoSorbents Issues Stockholder Letter and Reports Preliminary Fourth Quarter and Full Year 2022 Revenue
Cumulative CytoSorb treatments surpassed 195,000. Q4 2022 product sales rebounded from Q3 2022 low. Adjusted for constant currency, Core non-COVID 2022 product sales were within 5% of that achieved in 2021 and greater than 30% increased from pre-pandemic 2019 PRINCETON, N.J., Jan. 31, 2023 /PRNewswire/ — CytoSorbents Corporation (NASDAQ: CTSO), a leader in the treatment […]
VentureMed Group Raises Bridge Financing and Embarks on 2023 Objectives
MINNEAPOLIS, Jan. 31, 2023 /PRNewswire/ — VentureMed Group, Inc., a privately held medical device innovator in access management for arteriovenous (AV) fistulas and grafts and vessel preparation for interventional treatment of peripheral arterial disease (PAD) announced today that the company has raised bridge financing to support its 2023 objectives. Endeavour Vision and RiverVest Venture […]
Mindray Unveils Next-generation BeneHeart Defibrillation Solutions to Raise Standards for Resuscitation
SHENZHEN, China, Jan. 31, 2023 /PRNewswire/ — Mindray, a global leading medical device solutions provider, today announces the launch of next-generation BeneHeart series defibrillators, the BeneHeart D60 and D30. The new BeneHeart defibrillators aim to raise the standards for resuscitation with superior reliability, expert comprehensive diagnosis and monitoring tools, and introduce a […]