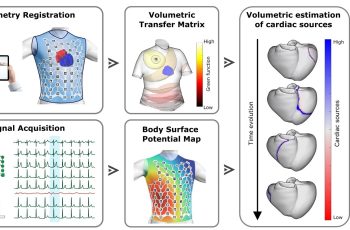
Imperative Care Launches Zoom 4S Catheter to Further Advance Company’s Zoom Stroke System for Ischemic Stroke Treatment
A Pivotal Step in Optimizing the ADAPT 2.0 Technique for Speed and Simplicity with a Single System Setup ADAPT 2.0 with the Zoom Stroke System CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced the commercial launch and first patient cases of the new Zoom 4S Catheter – an advanced aspiration thrombectomy […]