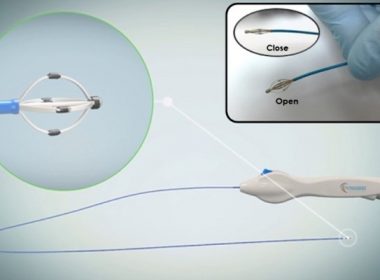
3D models will be used to simulate complex valve repairs in preparation for surgical interventions BOSTON, Jan. 5, 2022 /PRNewswire/ — BIOMODEX®, the leader in biorealistic haptic simulators for physician training and rehearsals, and the Montreal Heart Institute (MHI), a world-renowned, ultra-specialized cardiology center, announced they are partnering to develop a new cardiovascular training solution to […]
Coronary/Structural Heart
Confluent Medical Announces Significant Strategic Investment From TPG
TPG investment to fuel next stage of growth and expansion for Confluent, building on its strong foundation as a leading partner to medical device manufacturers SCOTTSDALE, Ariz. & SAN FRANCISCO–(BUSINESS WIRE)–Confluent Medical Technologies (“Confluent”), a leading materials science, development and manufacturing partner to medical device manufacturers (“OEMs”), today announced that […]
NewAmsterdam Pharma Doses First Patient in Phase 3 BROADWAY Trial of Obicetrapib in Adults with Heterozygous Familial Hypercholesterolemia (HeFH) and/or Established Atherosclerotic Cardiovascular Disease (ASCVD)
Study evaluates obicetrapib, a novel cholesteryl ester transfer protein (CETP) inhibitor, as an adjunct to diet and maximally tolerated lipid-lowering therapy Company is expected to complete enrollment in 2022 and announce data in the first quarter of 2024 NAARDEN, Netherlands–(BUSINESS WIRE)–NewAmsterdam Pharma (NAP), a clinical-stage company focused on the research […]
Pulnovo Medical Announced Results From PADN-CFDA Pivotal Trial For The Treatment Of Pulmonary Arterial Hypertension (PAH) Meet The Efficacy Primary Endpoint
SHANGHAI, Jan. 1, 2022 /PRNewswire/ — Pulnovo Medical Limited, a globally recognized device pioneer in treatment for cardiopulmonary disease, today announced the positive results from the Pulmonary Artery Denervation (PADN)-CFDA pivotal study, being the first global completed pulmonary hypertension treatment device RCT study. PADN is an innovative radiofrequency ablation technique in treating PH, was […]
Peijia Medical’s New Milestone Asia’s first successful clinical case using HighLife transseptal mitral valve replacement technology
SUZHOU, China, Dec. 30, 2021 /PRNewswire/ — On December 22, 2021, the first clinical case using HighLife transseptal mitral valve replacement (“TSMVR”) technology was successfully carried out in Asia. The implantation of Peijia’s Highlife TSMVR system was performed by Professor Mao Chen and his team of West China Medical Center of Sichuan University as part of a research clinical […]
SyMap Medical Ltd. Announces its Strategic Collaboration with Pythagoras Medical
SUZHOU, China, Dec. 29, 2021 /PRNewswire/ — SyMap Medical Ltd (SyMap) announced its strategic collaboration with Pythagoras Medical. With this win-win collaboration, SyMap has full rights to utilize all assets of Pythagoras, including their Renal Nerve Mapping System (ConfidenHT™, CE Mark Approval,) patents, trademarks and expertise. This collaboration will further enhance and consolidate […]
The Children’s Heart Foundation funds more than $1 million of new congenital heart defect research
To date, the foundation has funded $15 million of research into congenital heart defects–America’s most common birth defect. NORTHBROOK, Ill., Dec. 28, 2021 /PRNewswire/ — The Children’s Heart Foundation (CHF), the nation’s leading organization dedicated to funding congenital heart defect (CHD) research, will fund over $1,000,000 of research and scientific collaborations in 2021. Every 15 minutes […]
Siemens Healthineers to include Mentice VIST ® simulators with Corindus CorPath GRX Robotic PCI system (CorPath GRX) in China
Mentice and Siemens Healthineers China announce that Mentice VIST ® Virtual Patient simulators will be included with the sale of every Corindus CorPath GRX Robotic system (CorPath GRX) in China on an initial 3-year agreement, starting 2022. GOTHENBURG, Sweden, Dec. 27, 2021 /PRNewswire/ — Mentice, a world leader in simulation solutions for image-guided […]
Faraday Pharmaceuticals Announces Publication of Results from its Phase 2 Study of FDY-5301 for the Treatment of Reperfusion Injury Following a STEMI Heart Attack
To be Published in the International Journal of Cardiology SEATTLE, Dec. 27, 2021 (GLOBE NEWSWIRE) — Faraday Pharmaceuticals, Inc., a muscle health-focused biopharmaceutical company developing therapeutics to treat patients experiencing potentially life changing critical illness, announced today the publication of results from its Phase 2 trial of FDY-5301 for the […]
BridgeBio Pharma Reports Month 12 Topline Results from Phase 3 ATTRibute-CM Study
– ATTRibute-CM did not meet its primary endpoint at Month 12. Mean observed six-minute walk distance (6MWD) decline for the acoramidis and placebo arms were 9 meters and 7 meters, respectively. Both declines are similar to healthy elderly adults and less than prior untreated ATTR-CM cohorts – The company observed […]