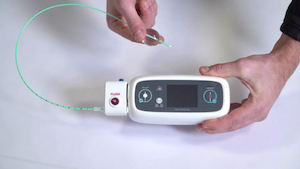
NIVA|HF is an investigational device designed to monitor the venous waveform, a novel physiologic signal, in heart failure patients. As a Breakthrough Device, NIVA|HF will receive pre-market developmental assistance and prioritized regulatory review from FDA. NIVA|HF is the first application of VoluMetrix’s proprietary venous waveform technology. NASHVILLE, Tenn., June 15, 2021 /PRNewswire/ […]
Coronary/Structural Heart
Establishing TAVR for the Treatment of Aortic Regurgitation(1)
JenaValve Marks the 100th Implant of the Trilogy™ Heart Valve System IRVINE, Calif., June 15, 2021 (GLOBE NEWSWIRE) — JenaValve Technology, Inc., developer, and manufacturer of a novel transcatheter aortic valve replacement (TAVR) system, is pleased to announce today the 100th global implant of the Trilogy™ Heart Valve System. This milestone […]
Cytokinetics Announces Completion of Enrollment in METEORIC-HF, The Second Phase 3 Clinical Trial of Omecamtiv Mecarbil in Patients With Heart Failure With Reduced Ejection Fraction
Top Line Results of METEORIC-HF Expected in Early 2022 Company Plans to Submit NDA in 2H 2021 Based on Positive Results of GALACTIC-HF SOUTH SAN FRANCISCO, Calif., June 15, 2021 (GLOBE NEWSWIRE) — Cytokinetics, Incorporated (Nasdaq: CYTK) today announced the completion of patient enrollment in METEORIC-HF (Multicenter Exercise Tolerance Evaluation of Omecamtiv Mecarbil Related to Increased Contractility […]
CMS Grants Transitional Pass-Through (TPT) Payment for Shockwave’s Coronary IVL
Provides Incremental Reimbursement in Hospital Outpatient Setting SANTA CLARA, Calif., June 11, 2021 (GLOBE NEWSWIRE) — Shockwave Medical, Inc. (NASDAQ: SWAV), a pioneer in the development of Intravascular Lithotripsy (IVL) to treat severely calcified cardiovascular disease, announced today that the Centers for Medicare & Medicaid Services (CMS) granted approval for a Transitional Pass-Through (TPT) payment for […]
GENinCode Announces Major US Commercialisation Partnership With EVERSANA
OXFORD, England, June 14, 2021 /PRNewswire/ — GENinCode UK Limited, the cardiovascular disease company focused on predictive genetics for the prevention of cardiovascular disease, announces its partnership with EVERSANA Life Sciences LLC (“EVERSANA”) as its launch and commercialisation partner to access the United States market for the GENinCode portfolio of polygenic cardiovascular disease (“CVD”) products focused […]
Caelum and Alexion Present Additional Phase 2 Data Reinforcing Safety and Tolerability of CAEL-101 in AL Amyloidosis at the European Hematology Association Congress 2021
– Exploratory biomarker analyses suggest possible cardiac disease improvements and renal response – BORDENTOWN, N.J. & BOSTON–(BUSINESS WIRE)–Caelum Biosciences and Alexion Pharmaceuticals, Inc. (NASDAQ:ALXN) today announced new Phase 2 safety and tolerability data for CAEL-101, a potentially first-in-class amyloid fibril targeted therapy, in combination with standard-of-care (SoC) therapy in patients with AL […]
Neurescue’s Breakthrough Intelligent Balloon Catheter FDA 510(k) Cleared for Hemorrhage Control and IDE Approved for Cardiac Arrest
Device is FDA IDE approved to start a clinical study in the U.S. to investigate a novel cardiac arrest treatment indication COPENHAGEN, Denmark–(BUSINESS WIRE)–Neurescue, a medical device company developing innovative cardiovascular solutions to improve the outcomes for emergency patients, today announced that the U.S. Food and Drug Administration (FDA) has […]
Peijia Medical Partners with inQB8 for US Incubator and Transcatheter Tricuspid Replacement (TTVR) Product
SUZHOU, China, June 9, 2021 /PRNewswire/ — Peijia Medical (HKEX:9996, or “Peijia“), a leading player in China for medical technology, announced a partnership with inQB8, a Boston based medical technology incubator, to explore innovative solutions for Structural Heart Disease. This partnership includes Peijia’s acquisition of a transcatheter tricuspid replacement technology (“TTVR”, or “inQB8 TTVR”) from inQB8, which is currently in […]
CVRx® Announces First Clinical Procedure with a New Ultrasound-Guided Implant Approach, the Latest Advancement in Barostim™ for the Treatment of Heart Failure Symptoms
MINNEAPOLIS, June 10, 2021 (GLOBE NEWSWIRE) — CVRx®, developer of the world’s first FDA-approved neuromodulation device to treat the symptoms of heart failure (HF), announced the completion of the first clinical procedure with the company’s new lead implantation approach. The novel ultrasound-guided technique is the latest advancement of CVRx’s Barostim™ Baroreflex Activation […]
vMap™’s Role in Optimizing Non-Invasive Radio-Ablation Therapy presented at ACC.21 and THRS 2021
A dual-center study from UCSD Health and Mills-Peninsula Sutter Health concluded that a simplified, non-invasive radio-ablation workflow, involving vMap™, was Feasible, Efficient, and Precise CARLSBAD, Calif.–(BUSINESS WIRE)–Vektor Medical, Inc. announced that a dual-center study reports that vMap™, as part of a new, non-invasive workflow, improved efficiency and precision for radio-ablation therapy […]