Santa Monica, CA, July 24, 2018 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (OTCQB: BSGM), a medical technology company developing a proprietary biomedical signal processing platform designed to address an unmet technology need for the $4.6 billion electrophysiology (EP) marketplace, was featured in The Silicon Review’s list of Top 50 Innovative […]
Author: Ken Dropiewski
HeartStitch® Presents Initial Clinical Results Of New Tricuspid Valve Repair At CSI Frankfurt Clinical Congresses
FRANKFURT, Germany, July 24, 2018 /PRNewswire/ — HeartStitch® has presented initial clinical data of its revolutionary tricuspid valve repair technique using only surgical sutures and pledgets to repair and promote remodeling of the tricuspid valve in 30 patients suffering from tricuspid regurgitation (TR). Prof. Anthony Nobles, Chairman, CEO and Chief Clinical Officer of HeartStitch®, Inc. […]
Nation’s Top Heart Disease Patient Advocates Create Task Force Aimed at Improving Access, Research, and Awareness on Heart Valve Disease Detection and Treatment
WASHINGTON, July 24, 2018 /PRNewswire/ — The nation’s leading heart disease patient advocates announced today the launch of the Heart Valve Disease Policy Task Force, a coalition of non-profit organizations uniting for a common voice in support of improved access, research, and awareness on heart valve disease detection and treatment. The Heart Valve Disease […]
Hancock Jaffe Laboratories Receives Ethics Committee Approval for First-in-Human VenoValve Study
IRVINE, Calif., July 24, 2018 (GLOBE NEWSWIRE) — Hancock Jaffe Laboratories, Inc. (Nasdaq:HJLI) (Nasdaq:HJLIW), a company specializing in bioprosthetic medical devices to establish improved standards of care for treating cardiac and vascular diseases, today announced that it has received approval from the Ethics Committee at Fundación Santa Fe de Bogotá […]
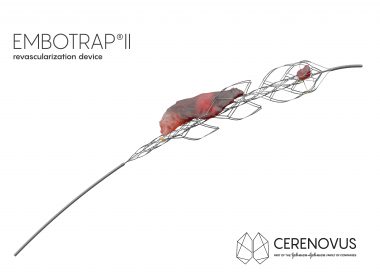
First Ischemic Stroke Patients Treated With EMBOTRAP II Revascularization Device Since Commercial Availability In U.S.
SAN FRANCISCO, July 24, 2018 /PRNewswire/ — CERENOVUS, part of the Johnson & Johnson Medical Devices Companies, announced the first patients have been treated with its new EMBOTRAP II Revascularization Device since it became commercially available in the U.S. EMBOTRAP II is a next generation stent retriever used to capture and remove life-threatening blood clots […]
OPTALYSE PE Results Published in JACC: Cardiovascular Interventions
BOTHELL, Wash.–(BUSINESS WIRE)–BTG plc (LSE: BTG), the global healthcare company, highlighted the publication of OPTALYSE PE trial results in JACC: Cardiovascular Interventions. The published findings further confirm that bilateral pulmonary embolism (PE) treated in as little as 2 hours with EKOSⓇ Acoustic Pulse Thrombolysis™ therapy shows significant improvement in RV/LV ratio and […]
Metactive Presents Results from Nonclinical Research Study Treating Peripheral Arteries
FAIRWAY, Kan.–(BUSINESS WIRE)–Metactive Medical Inc. (Metactive), a medical device company developing innovative products for the treatment of neurovascular and peripheral vascular diseases, announced today that Dr. Kieran Murphy, Professor, Vice Chair and Director of Research in the Department of Medical Imaging at the University of Toronto, presented new nonclinical results from […]
Metactive Presents Results from Nonclinical Research Study Treating Carotid Artery Aneurysms
FAIRWAY, Kan.–(BUSINESS WIRE)–Metactive Medical Inc. (Metactive), a medical device company developing innovative products for the treatment of neurovascular and peripheral vascular diseases, announced today that Dr. Kieran Murphy, Professor, Vice Chair, and Director of Research in the Department of Medical Imaging at the University of Toronto, presented new nonclinical results from […]
InspireMD Announces Opening of First Two CGuard™ EPS Centers of Excellence
Tel Aviv, Israel, July 23, 2018 (GLOBE NEWSWIRE) — InspireMD, Inc. (NYSE AMER: NSPR), developer of the CGuard™ Embolic Prevention System (EPS) for the prevention of stroke caused by the treatment of carotid artery disease, today announced that it has established its first two Centers of Excellence (COE) at leading […]
Intra-Cellular Therapies Announces Publication Highlighting ITI-214 Mechanism of Action and Describing Positive Results in Preclinical Models of Heart Failure
NEW YORK, July 23, 2018 (GLOBE NEWSWIRE) — Intra-Cellular Therapies, Inc. (Nasdaq:ITCI) today announced the online publication of a report in the journal Circulation describing the mechanism of action of its phosphodiesterase (PDE) Type 1 inhibitor, ITI-214, and its potential use for the treatment of heart failure. In these preclinical studies, Johns […]