Mar 31, 2017 – IRVINE, Calif.–(BUSINESS WIRE)– Endologix, Inc. (Nasdaq:ELGX), a developer and marketer of innovative treatments for aortic disorders, announced today that the first patients were treated in the Expanding Patient Applicability with Polymer Sealing Ovation Alto Stent Graft (ELEVATE) IDE clinical study, the Company’s pivotal clinical trial to […]
Author: Ken Dropiewski
Medtronic Initiates Global Trial Evaluating Cryoablation to Treat Persistent Atrial Fibrillation
Press Release DUBLIN – March 30, 2017 – Medtronic plc (NYSE: MDT) today announced first enrollments in the STOP Persistent AF clinical trial. The trial will evaluate the safety and effectiveness of a pulmonary vein isolation-only (PVI) strategy for treating patients with persistent atrial fibrillation (AF), using the Arctic Front Advance(TM) Cardiac […]
Headcount Balloons as Abiomed (ABMD) Opens Doors to Its New Massachusetts HQ
Abiomed Marks Grand Opening of Newly-Expanded Headquarters in Danvers, Massachusetts – Governor Baker joins heart recovery leader for grand opening of new manufacturing and training space that represents major investment in Massachusetts DANVERS, Mass., March 30, 2017 (GLOBE NEWSWIRE) — Massachusetts Governor Charlie Baker today joined Abiomed – a leading […]
Cardiovascular Systems Snags FDA Nod for Its Diamondback 360 Coronary Orbital Atherectomy System (OAS)
Cardiovascular Systems, Inc. Receives Approval for the Diamondback 360® Coronary Orbital Atherectomy System (OAS) Micro Crown in the United States OAS Micro Crown Approved to Treat Severely Calcified Coronary Lesions Only Atherectomy Device Designed to Both Pilot Tight Lesions and Treat Up to 4mm Vessels with a Single Device ST. […]
Utah’s Merit Medical (MMSI) Prices $136.5 Million Offering
Merit Medical Announces Closing of Public Offering of Common Stock SOUTH JORDAN, Utah, March 29, 2017 (GLOBE NEWSWIRE) — Merit Medical Systems, Inc. (NASDAQ:MMSI) (“Merit”), a leading manufacturer and marketer of proprietary disposable devices used in interventional, diagnostic and therapeutic procedures, particularly in cardiology, radiology and endoscopy, today announced that […]
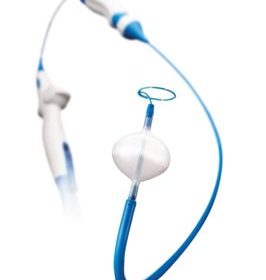
Boston Scientific agrees to acquire Symetis
MARLBOROUGH, Mass., March 30, 2017 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced a definitive agreement to acquire Symetis SA, a privately-held Swiss structural heart company focused on minimally-invasive transcatheter aortic valve implantation (TAVI) devices, for $435 million in up-front cash. The Symetis portfolio includes the ACURATE TA™ and ACURATE neo/TF valve* […]
Study Suggests Marijuana Use Can Bring a Greater Risk of Cardiovascular Disease
By Ken Dropiewski, Ken@Prime-Core.com According to LiveScience, a study conducted by the Einstein Medical Center in Philadelphia suggests that marijuana users have an increased risk of a number of cardiovascular diseases, including strokes and heart failure. The study did have some limited parameters, pointing to the need for more research. The […]
New Guidelines Support Use of Cardiac Monitoring for Patients with Unexplained Fainting
Press Release American College of Cardiology, American Heart Association and Heart Rhythm Society Jointly Issue First-Ever Syncope Guidelines that Reinforce Benefits of Continuous Cardiac Monitoring DUBLIN – March 24, 2017 – Newly published guidelines from the American College of Cardiology (ACC), the American Heart Association (AHA), and the Heart Rhythm […]
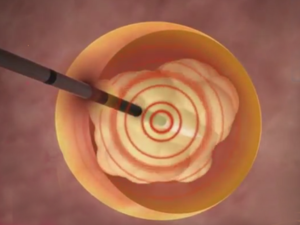
Surgery Without the Knife: Mirabilis Medical Announces European Approval for Non-Invasive Uterine Fibroid Treatment
BOTHELL, Wash., March 23, 2017 /PRNewswire/ — Mirabilis Medical, a Seattle-area developer of advanced medical technology for non-invasive surgery, announced today CE Mark authorization for marketing of the Mirabilis System for treatment of uterine fibroids throughout the European Union. The company simultaneously announced that it had received approval from the US […]
AngioDynamics Receives CE Mark Certification for The Solero Microwave Tissue Ablation System
ALBANY, N.Y., March 14, 2017 (GLOBE NEWSWIRE) — AngioDynamics (Nasdaq:ANGO), a leading provider of innovative, minimally invasive medical devices for vascular access, peripheral vascular disease and oncology/surgery, today announced it has received CE Mark certification for the Solero Microwave Tissue Ablation (MTA) System. The Solero MTA System and Accessories are […]