ALAMEDA, Calif., Aug. 24, 2017 /PRNewswire/ — Penumbra, Inc. (NYSE: PEN) today announced that its management team is scheduled to present at the following upcoming investor conferences: Event: Wells Fargo 2017 Healthcare Conference Location: Boston, MA at the Westin Boston Waterfront Date: Thursday, September 7, 2017 Time: 1:40pm ET / 10:40am PT Event: Morgan […]
Author: Ken Dropiewski
Ra Medical Looks for Potential IPO in Early 2018
Ra Medical finally received FDA approval this year after three years, now they will be shooting for an IPO. Ra Medical CEO Irwin aims for Feb 2018 IPO AUGUST 24, 2017 BY FINK DENSFORD, MassDevice In May, Ra Medical received a nod from the FDA for its Dabra arteriosclerosis laser, clearing it for use in the […]
Medtronic Receives CE Mark for Attain Stability(TM) Quad MRI SureScan(TM) Active-Fixation Heart Lead
DUBLIN – August 21, 2017 – Medtronic plc (NYSE: MDT) announced it has received CE (Conformité Européene) Mark for the Attain Stability(TM) Quad MRI SureScan(TM) left heart lead. Paired with Medtronic quadripolar cardiac resynchronization therapy-defibrillators (CRT-D) and -pacemakers (CRT-P), the Attain Stability Quad lead features novel, active-fixation technology that is designed […]
Implanted Cardiac Device: Newest Ransomware Target
by Ken Dropiewski, Prime-Core Executive Search (ken@prime-core.com) It has the makings of a plot for a blockbuster movie: a high ranking government official or world leader assassinated by a nefarious hacker who remotely accesses an implanted cardiac device. Plot lines and prominent world leaders notwithstanding, cyber-security risks for implantable cardiac […]
Malin Announces U.S. FDA Approval for New Hourglass™ Peripheral Embolisation Plug
DUBLIN–(BUSINESS WIRE)–Malin Corporation plc. (ISE:MLC, “Malin”), an Irish based and globally operating life sciences company, today announced that its EMBA device, known as the Hourglass™ Peripheral Embolisation Plug, was granted U.S. FDA 510(k) clearance to commence marketing in the US. The Hourglass™ peripheral embolisation plug represents a breakthrough in peripheral […]
Getinge Announces Full U.S. Availability Of Pulsar-18 Self-Expanding Stent From BIOTRONIK For Patients With Peripheral Artery Disease
WAYNE, N.J., Aug. 22, 2017 /PRNewswire/ — Getinge, a leading global provider of innovative medical technology, today announces the full U.S. market release of the Pulsar®-18 stent from BIOTRONIK. Pulsar-18 is the only available self-expanding stent for blocked superficial femoral arteries with a 4-French (4F) delivery system. Getinge currently distributes BIOTRONIK’s portfolio of […]
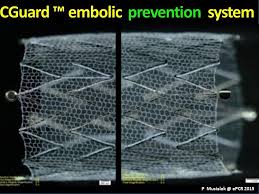
InspireMD Shares CGuard EPS Video Testimonials From European Key Opinion Leaders
TEL AVIV, ISRAEL–(Marketwired – August 22, 2017) – InspireMD, Inc. (NYSE American: NSPR) (NYSE MKT: NSPR) (NYSE American: NSPR.WS) (NYSE MKT: NSPR.WS) (“InspireMD” or the “Company”), a leader in embolic prevention systems (EPS) / thrombus management technologies and neurovascular devices, today announced it has posted video testimonials about its CGuard™ Embolic Prevention System from […]
InspireMD Announces Notification Of NYSE AMERICAN Listing Deficiency And Expectation To Regain Compliance
TEL AVIV, ISRAEL–(Marketwired – August 22, 2017) – InspireMD, Inc. (NYSE American: NSPR) (NYSE MKT: NSPR) (“InspireMD” or the “Company”), a leader in embolic prevention systems (EPS) / thrombus management technologies and neurovascular devices, today announced it received a letter from the NYSE American on August 17, 2017 indicating that InspireMD does not […]
BioVentrix Announces First Patient Enrolled In IDE Study Of The Revivent TC Transcatheter Ventricular Enhancement Treatment For Ischemic Cardiomyopathy
SAN RAMON, Calif. and CAMBRIDGE, England, Aug. 21, 2017 /PRNewswire/ — BioVentrix, Inc. a pioneer of technologies and procedures for less invasive treatment of heart failure (HF), today announced enrollment of the first patient in the international arm of the ALIVE pivotal clinical trial. The trial is designed to demonstrate the safety and effectiveness of […]
Cardiome Provides U.S. Regulatory Update For BRINAVESS
VANCOUVER, Aug. 21, 2017 /PRNewswire/ – Cardiome Pharma Corp. (NASDAQ: CRME/ TSX:COM) today announced that it has received a response from the U.S. Food and Drug Administration (FDA) regarding the regulatory path for BRINAVESS® (vernakalant hydrochloride, IV), the Company’s antiarrhythmic drug for the rapid conversion of recent onset atrial fibrillation (AF). In its […]