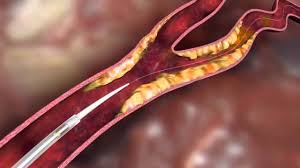
TEL AVIV, ISRAEL–(Marketwired – August 03, 2017) – InspireMD, Inc. (NYSE MKT: NSPR) (NYSE MKT: NSPR.WS) (“InspireMD” or the “Company”), a leader in embolic prevention systems (EPS) / thrombus management technologies and neurovascular devices, today announced that an endovascular interventional procedure featuring the CGuard™ EPS performed by the team of Dr. Anibal Damonte, Interventional […]
Author: Ken Dropiewski
RTI Surgical® Divests Cardiothoracic Closure Business to A&E Medical Corporation for up to $60 Million
ALACHUA, Fla.–(BUSINESS WIRE)–RTI Surgical Inc. (RTI) (Nasdaq: RTIX), a global surgical implant company, today announced that it has sold its cardiothoracic closure business to A&E Advanced Closure Systems, LLC (A&E Medical), a leading cardiovascular medical device OEM and a portfolio company of Vance Street Capital LLC, for $54 million in cash […]
Boston Scientific’s Distribution Option Lapses
SAN DIEGO, Aug. 03, 2017 (GLOBE NEWSWIRE) — REVA Medical, Inc. (ASX:RVA) (“REVA” or the “Company”) announces the expiration of Boston Scientific Corporation’s (“BSC”) exclusive right to negotiate for distribution of REVA’s coronary and peripheral bioresorbable scaffolds. BSC’s right to negotiate was available under a 2007 agreement that would have […]
University of Maryland Medicine Establishes Nation’s First Center For Cardiac Xenotransplantation Research With $24 Million Grant And Addition Of Top Transplant Surgeon-Scientists
BALTIMORE, Aug. 3, 2017 /PRNewswire-USNewswire/ —Stephen Bartlett, MD, chair of the Department of Surgery at the University of Maryland School of Medicine, and UM SOMDean E. Albert Reece, MD, PhD, MBA, announced today that a recent $24 million grant from United Therapeutics Corporation will establish the first and only center for cardiac xenotransplantation research in the […]
SHS invests in innovative heart valve-repair start-up CoreMedic
Tuebingen, 27. July 2017. SHS Gesellschaft für Beteiligungsmanagement is investing in CoreMedic, a start-up developing innovative, transfemoral repair systems to treat heart valve malfunctions. SHS is investing from its fourth fund generation to finance CoreMedic’s further growth and the clinical application of its innovative products. Mitral heart valve regurgitation (MR) […]
Cardiovascular Systems Reports Fiscal 2017 Fourth-Quarter Financial Results
ST. PAUL, Minn.–(BUSINESS WIRE)–Cardiovascular Systems, Inc. (NASDAQ: CSII): Conference Call Scheduled for Today, August 2, 2017, at 3:45 PM CT (4:45 PM ET) Revenues of $52.9 million grew 9% compared to fourth quarter last year Gross margin increased to 81.7% from 79.7% in the prior-year period Net income was $0.8 […]
Endologix Reports Second Quarter 2017 Financial Results
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (NASDAQ:ELGX), a developer and marketer of innovative treatments for aortic disorders, today announced financial results for the second quarter ended June 30, 2017. “While we are pleased with the overall results in the second quarter, the recovery in AFX®2 sales in the U.S. has been slower […]
4C Medical’s Novel Mitral Regurgitation Therapy Highlighted at CVI 2017 Innovation Summit
NEWS PROVIDED BY 4C Medical Technologies, Inc. 07:04 ET BROOKLYN PARK, Minn., Aug. 3, 2017 /PRNewswire/ — 4C Medical Technologies, Inc. (4C Medical), a developer of minimally invasive therapies for structural heart disease, today announced that its medical device therapy for mitral regurgitation (MR) was featured in the Innovation Summit at Cardiovascular Innovations (CVI) 2017 […]
Medtronic (MDT) Bags Approval for Avalus Valve in U.S. and Europe
DUBLIN – August 2, 2017 – Medtronic plc (NYSE: MDT) today announced CE (Conformité Européenne) mark and U.S. Food and Drug Administration (FDA) approval of its new Avalus(TM) pericardial aortic surgical valve for the treatment of aortic valve disease. The Avalus valve leverages proven surgical bioprosthetic valve concepts with added features designed to […]
Boca Raton Regional Hospital Using New Technology To Map Irregular Heart Rhythms Non-Invasively
NEWS PROVIDED BY Boca Raton Regional Hospital Aug 01, 2017, 12:24 ET BOCA RATON, Fla., Aug. 1, 2017 /PRNewswire-USNewswire/ — Boca Raton Regional Hospital is the first in Florida and one of only five centers nationally to non-invasively map irregular heartbeats in patients with chronic atrial fibrillation and other arrhythmias who have not responded adequately […]