ALAMEDA, Calif., July 25, 2017 /PRNewswire/ — Penumbra, Inc.(NYSE: PEN) today announced that it will host a conference call to discuss financial results for the second quarter 2017 after market close on Tuesday, August 8, 2017 at 4:30 PM Eastern Time. A press release with second quarter 2017 financial results will be issued after market close […]
Author: Ken Dropiewski
Corindus Vascular Robotics To Report Second Quarter 2017 Financial Results On August 8, 2017
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. [NYSE MKT: CVRS], a leading developer of precision vascular robotics, today announced that it will release financial and business results for the second quarter of 2017 after the close of trading on Tuesday, August 8, 2017. The Company’s management team will host a corresponding conference […]
Medtronic (MDT) First To Receive Health Canada Licence For MR-Conditional Cardiac Resynchronization Therapy-Defibrillators
BRAMPTON, Ontario, July 26, 2017 (GLOBE NEWSWIRE) — Medtronic Canada, a subsidiary of Medtronic plc, today announced it has received a Health Canada licence and is launching its first and only magnetic resonance imaging (MRI) conditional cardiac resynchronization therapy defibrillators (CRT-Ds) for heart failure. Heart failure causes or contributes to […]
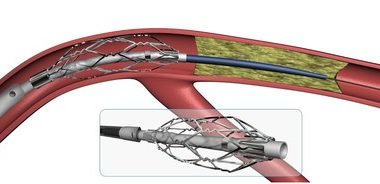
SurModics (SRDX) Receives IDE Approval To Initiate Pivotal Trial Of The Surveil Drug-Coated Balloon
EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–Surmodics, Inc. (Nasdaq: SRDX), a leading provider of medical device and in vitro diagnostic technologies, today announced it has received an investigational device exemption (IDE) from the U.S. Food and Drug Administration (FDA) to initiate a pivotal clinical trial of the SurVeil™ drug-coated balloon (DCB). The randomized […]
BioCardia Completes Roll-In Cohort In Pivotal Phase III CardiAMP Heart Failure Trial
SAN CARLOS, Calif.–(BUSINESS WIRE)–BioCardia®, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced completion of treatment for the 10-patient roll-in cohort for the pivotal Phase III CardiAMP Heart Failure Trial. A pre-specified review of the 30-day outcomes in this cohort by the Data […]
Penumbra initiates recall of 3D Revascularation Device
Penumbra Inc. Recalls 3D Revascularization Device Due to Wire Material That May Break or Separate During Use Penumbra Inc. is recalling the Penumbra 3D Revascularization device because there is a risk of the delivery wire breaking or separating during use. Fractured pieces of the delivery wire could be left inside […]
The Cath Lab of the Future is Here
JULY 26, 2017 BY Ken Dropiewski (ken@prime-core.com) In March, Miami Cardiac & Vascular Institute announced completion of its state-of-the-art cath lab, with the first next ten image-guided therapy system was being designed to reduce radiation exposure, decrease prep and procedure time and visually eliminate human error in the preparation and treatment […]
ABBOTT INITIATES CLINICAL TRIAL OF THREE-MONTH DUAL ANTIPLATELET THERAPY FOLLOWING IMPLANTATION WITH XIENCE CORONARY STENT
PRESS RELEASE ABBOTT PARK, Ill., July 25, 2017 — Abbott today announced that the first patient has been enrolled in a clinical study evaluating the short-term use of common blood thinning medicines, called dual antiplatelet therapy (DAPT), after receiving a XIENCE everolimus-eluting coronary stent. The study, called XIENCE Short DAPT, […]
Roxwood Medical Announces Agreement With Abbott (ABT) For Distribution Of Products In U.S.
REDWOOD CITY, Calif., July 25, 2017 /PRNewswire/ — Roxwood Medical Inc., a leading provider of advanced cardiovascular specialty catheters, today announced it has entered into an exclusive agreement with Abbott for distribution of Roxwood products in the United States. “This partnership with Abbott’s vascular business is a major milestone for Roxwood that allows far […]
Pulsar and Neuravi Now Part of J&J’s New Cerenovous Neurovascular Business
Johnson & Johnson rolls Pulsar Vascular, Neuravi into new Cerenovus neurovascular biz JULY 25, 2017 BY BRAD PERRIELLO – MassDevice Johnson & Johnson (NYSE:JNJ) said yesterday that it’s medical device division is rolling the Pulsar Vascular and Neuravi acquisitions into a new neurovascular business dubbed Cerenovus. The New Brunswick, N.J.-based healthcare giant said the new brand, unveiled at […]