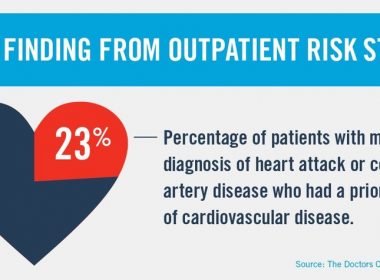
NEWS PROVIDED BY CRICO Strategies 27 Jun, 2017, 10:33 ET BOSTON, June 27, 2017 /PRNewswire/ — When doctors miss a diagnosis of cardiovascular disease in an outpatient setting, the patient often has conventional risk factors for coronary artery disease, a groundbreaking study reveals. The research, published online today in The Joint Commission Journal on Quality […]
Author: Ken Dropiewski
What is AR and How is it Disrupting Healthcare?
JUNE 28, 2017 BY KEN DROPIEWSKI (KEN@PRIME-CORE.COM) Real-time information is critical while performing a procedure. Doctors often require data from outside sources to proceed in a difficult situation. But obtaining such information during an intervention can be problematic and may even place the patient at risk. Augmented reality (AR) is one technology that […]
Philips receives FDA 510(k) clearance to market multiple new applications on its IntelliSpace Portal platform for Radiology
AMSTERDAM, June 27, 2017 /PRNewswire/ — Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market IntelliSpace Portal 9.0 and a range of innovative applications for Radiology in the U.S. The latest […]
Boston Scientific (BSX) Outlines Growth Strategy
MARLBOROUGH, Mass., June 27, 2017 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) is hosting a meeting with the investment community today in New York City to provide an overview of its business and long-term growth strategies. Chairman of the Board and Chief Executive Officer Mike Mahoney will discuss how the […]
Acessa Health Inc. Acquires The Acessa System For Treatment Of Symptomatic Uterine Fibroids
AUSTIN, Texas–(BUSINESS WIRE)–Acessa Health Inc. announced today that it has acquired the Acessa® System for the treatment of symptomatic uterine fibroids, following the close of an asset purchase agreement with Halt Medical Inc. Concurrent with the acquisition, Acessa Health completed the first closing of a planned $30 million Series A […]
BioSig Technologies Release: Novel Findings Obtained With The PURE EP System To Be Presented At American Heart Association’s BCVS Scientific Sessions 2017
Minneapolis, MN, June 26, 2017 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (OTCQB: BSGM), a medical device company developing a proprietary platform designed to address an unmet technology need for the $4+ billion electrophysiology (EP) marketplace, today announced that the American Heart Association’s 13th Annual Basic Cardiovascular Sciences (BCVS) 2017 Scientific […]
Heart Endovascular & Rhythm of Texas Begins First Commercial, FDA-Cleared, In-Patient Use of DABRA™ to Treat Peripheral Artery Disease
SAN ANTONIO–(BUSINESS WIRE)–Today, the Heart Endovascular & Rhythm of Texas announced its latest collaboration with Ra Medical Systems, makers of cardiovascular and dermatology catheters and excimer lasers, to use its groundbreaking DABRA™ System for the treatment of Peripheral Artery Disease (PAD) in order to combat the growing rise of diabetic […]
Teleflex (TFX) Announces 510(k) Clearance and Global Launch of Twin-Pass® Torque Dual Access Catheter
WAYNE, Pa.–(BUSINESS WIRE)–Teleflex Incorporated (NYSE: TFX), a leading global provider of medical technologies for critical care and surgery, has announced 510(k) clearance by the Food and Drug Administration and both U.S. and international commercial launch of the Twin-Pass Torque Dual Access Catheter. “The Twin-Pass Torque Catheter builds on Vascular Solutions’ […]
BioImage-2 Starts: Ultrasound Imaging for Cardiac Risk Prediction Looks at Disease Progression
NEW YORK, June 20, 2017 /PRNewswire/ — Mount Sinai Heart and BioImage-2 LLC, today announced start of the BioImage-2 study. The BioImage-2 study investigates the progression of vascular atherosclerotic plaque in patients who previously participated in the BioImage study. That landmark study recruited 7,687 volunteer participants from Humana members in Greater […]
Millar, Inc. Sells Inca® Pressure-Volume (PV) Loop System to the University of Colorado to Support LVAD Research
HOUSTON, June 22, 2017 /PRNewswire/ — Millar, Inc. is pleased to announce that the University of Colorado Anschutz Medical Campus has purchased the CD Leycom® Inca® Pressure-Volume (PV) Loop System – sold in North America exclusively by Millar, Inc. – to better understand how left ventricular assist devices (LVADs) impact ventricular function […]