PARIS & EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–ACIST Medical Systems Inc., a Bracco Group Company, today announced results from the ACIST-FFR Study (Assessment of Catheter-based Interrogation and Standard Techniques for Fractional Flow Reserve measurement) demonstrating the consistent and correlative performance of the Navvus® MicroCatheter compared to standard pressure wire systems. ACIST-FFR is […]
Author: Ken Dropiewski
Elixir Medical Announces Outstanding 5-Year Clinical Data For CE Mark-Approved Desolve Novolimus Eluting Bioresorbable Coronary Scaffold System
MILPITAS, Calif.–(BUSINESS WIRE)–Elixir Medical Corporation, a developer of products that combine state-of-the-art medical devices with advanced pharmaceuticals, today announced excellent 5-year clinical data from the DESolve Nx international pivotal clinical trial for the CE Mark-approved, fully bioresorbable DESolve® Novolimus Eluting Coronary Scaffold System. Stefan Verheye, MD, PhD, ZNA Middleheim Hospital, […]
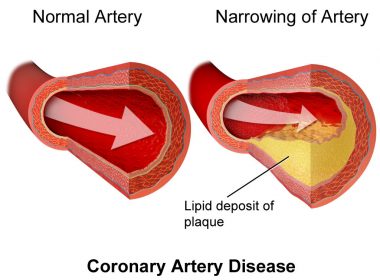
What is the Significance of Lipid-rich Plaque and Should it Influence Targets for PCI?
By Ken Dropiewski Lipid-rich plaque in non-culprit lesions has long been associated with subsequent cardiac events. Unlike culprit lesions, known to be the cause of AMI, non-culprit lesions including those with a core of lipid-rich plaque are often left untreated during an intervention. The fact that these lesions may lead […]
W. L. Gore & Associates Announces Positive Results From REDUCE Clinical Study For PFO Closure
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) announces positive results from its REDUCE Study assessing closure of patent foramen ovale (PFO) for the reduction of recurrent ischemic stroke and new brain infarct. The data were shared May 16 at the European Stroke Organisation Conference (ESOC) in Prague, Czech […]
Lombard Medical (LMT.L) Announces First Patient Enrolled And Treated In The ALTITUDE Registry For The Altura Stent Graft System
OXFORDSHIRE, U.K.–(BUSINESS WIRE)–Lombard Medical, Inc. (NASDAQ: EVAR), a developer, manufacturer and marketer of endovascular aortic aneurysm repair products, today announced that it has enrolled and treated the first patient in its global registry to evaluate its Altura® Endograft System. The ALTITUDE registry (ALTura Impact on the Treatment of Abdominal Aortic […]
Shockwave Medical Snags CE Mark for Coronary Lithoplasty System
FREMONT, Calif.–(BUSINESS WIRE)–Shockwave Medical, a pioneer in the treatment of calcified cardiovascular disease, today announced conformité européenne (CE) Mark for the company’s Coronary Lithoplasty® System for the treatment of calcified plaque in conjunction with stenting in patients with coronary artery disease. The Shockwave Medical Coronary Lithoplasty System is an innovative […]
New Study Shows Medtronic Insertable Cardiac Monitors Detect High Rate of Atrial Fibrillation in Previously Undiagnosed High-Risk Patients
By GlobeNewswire DUBLIN and CHICAGO – May 12, 2017 -Medtronic plc (NYSE:MDT) today announced results from a new clinical study showing Medtronic Insertable Cardiac Monitors (ICM) detected a high incidence of atrial fibrillation (AF) in patients previously undiagnosed but suspected to be at high-risk for AF and stroke. These data […]
Healthcare Veteran John Fletcher Joins MRI Interventions (MRIC)’ Board of Directors
IRVINE, Calif., May 11, 2017 (GLOBE NEWSWIRE) — MRI Interventions, Inc. (OTCQB:MRIC) is pleased to announce that John Fletcher has been appointed to MRI Interventions’ Board of Directors. Mr. Fletcher, founder and managing partner of Fletcher Spaght, Inc., brings more than 30 years of experience in healthcare with an emphasis […]
Shockwave Medical Taps Former HeartWare (HTWR) Exec as New CEO
FREMONT, Calif.–(BUSINESS WIRE)–Shockwave Medical, a pioneer in the treatment of calcified cardiovascular disease, today announced the appointment of Doug Godshall as president and CEO. “We are excited to welcome an executive of Doug’s caliber and experience to the Shockwave team at this important time in the company’s development” Godshall was […]
BioTelemetry Announces The Start Of The Tender Offer Period For LifeWatch Corp Shares
MALVERN, Pa. and ZUG, Switzerland, May 10, 2017 (GLOBE NEWSWIRE) — BioTelemetry, Inc. (NASDAQ:BEAT), the leading wireless medical technology company focused on the delivery of health information to improve quality of life and reduce cost of care, announced today the start of the main offer period of the tender offer […]