By Ken Dropiewski In the field of stroke treatment, there is a saying “time is brain.” This is because every minute with a clot a patient loses millions of brain cells. Stroke patients living near one to the nation’s Comprehensive Stroke Centers (CSC) are luckier than most. In 2015, 81 […]
Author: Ken Dropiewski
Corindus Vascular Robotics Reports First Quarter 2017 Results
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. [NYSE MKT: CVRS], a leading developer of precision vascular robotics, today reported financial results for the quarter ended March 31, 2017. Recent Highlights and Accomplishments Realized revenue of $0.8 million in the first quarter 2017 Backlog of an additional $3.0 – $3.5 million in […]
NaviGate Cardiac Structures, Inc. (“NCSI”) reports first heterotopic transcatheter GATE™ tricuspid valved stent successful implantation
LAKE FOREST, Calif.–(BUSINESS WIRE)–NaviGate Cardiac Structures Inc. (“NCSI”) announced today that the GATE™ catheter-guided tricuspid atrioventricular valved stent (AVS) was implanted through the jugular vein one week ago in a patient suffering from severe tricuspid regurgitation stemming from two failed tricuspid annuloplasty rings that were unable to maintain the patient’s […]
Abiomed (ABMD) Introduces 3rd Generation Impella CP® Heart Pumps At SCAI 2017
DANVERS, Mass., May 09, 2017 (GLOBE NEWSWIRE) — Abiomed, Inc. (NASDAQ:ABMD), a leading provider of breakthrough heart support and recovery technologies, announced the debut of the 3rd Generation Impella CP heart pump at the Annual meeting of the Society for Cardiovascular and Angiography Interventions (SCAI 2017) in New Orleans, LA. […]
Cook Medical Launches Acrobat 2 Calibrated Tip Wire Guide
BLOOMINGTON, Ind.–(BUSINESS WIRE)–Cook Medical’s Endoscopy business unit redefines access with the introduction of their latest wire guide, the next generation Acrobat® 2 Calibrated Tip Wire Guide. The Acrobat 2 provides the tip flexibility needed for ductal navigation. “We’re very excited to launch the Acrobat 2 wire guide” “We’re very excited […]
BioSig Technologies Release: PURE EP System Featured In The Journal Of Innovations In Cardiac Rhythm Management
Minneapolis, MN, May 09, 2017 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (OTCQB: BSGM), a medical device company developing a proprietary platform designed to address an unmet technology need for the $4+ billion electrophysiology (EP) marketplace, today announced that the Company’s co-authored manuscript with physicians from Mayo Clinic and Harvard Brigham […]
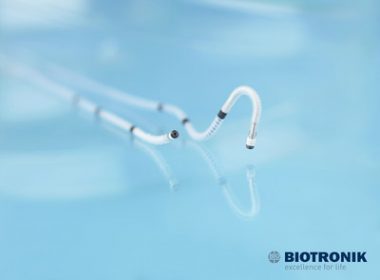
FDA Green Lights BIOTRONIK’s Sentus ProMRI Quadripolar Left Ventricular Lead
LAKE OSWEGO, Oregon, May 8, 2017 /PRNewswire/ — BIOTRONIK today announced FDA approval and the launch of Sentus ProMRI®, the thinnest quadripolar left ventricular lead available in the United States. This introduction completes BIOTRONIK’s second-generation ProMRI lead portfolio, which also includes Solia ProMRI and Plexa ProMRI. Sentus ProMRI is approved for […]
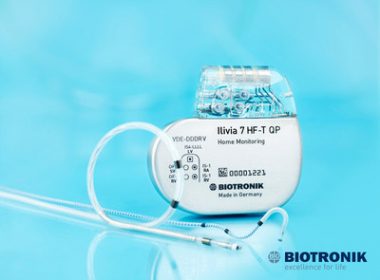
BIOTRONIK Nabs FDA OK of MultiPole Pacing with ProMRI: 360° Solutions for Patients With Heart Failure
LAKE OSWEGO, Ore., May 8, 2017 /PRNewswire/ — BIOTRONIK today announced FDA approval of the company’s MultiPole Pacing (MPP) technology, providing physicians with additional treatment options for heart failure patients who have been non-responsive to cardiac resynchronization therapy (CRT).1 MPP will be available on new BIOTRONIK CRT defibrillator (CRT-D) systems for […]
Boston Scientific (BSX) Snags FDA Approval for Resonate Family of High-Voltage Devices
MARLBOROUGH, Mass., May 9, 2017 /PRNewswire/ — Boston Scientific (NYSE: BSX) has received U.S. Food and Drug Administration (FDA) approval for the Resonate family of implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy defibrillator (CRT-D) systems. The approval includes new features in the Resonate devices including SmartCRT technology with Multisite Pacing […]
Murj Launches Cardiac Device Data Management Platform at Heart Rhythm Society 2017
SANTA CRUZ, Calif.–(Business Wire)–Murj, Inc., a digital health company dedicated to helping clinicians streamline care for patients with implantable cardiac devices, today announced general availability of the company’s cloud-based, HIPAA-compliant application that consolidates data from all implantable cardiac device (CIED) types and manufacturers into an elegant, robust platform that delivers […]