QUEENSBURY, N.Y.–(BUSINESS WIRE)–Delcath Systems, Inc. (Nasdaq: DCTH), (“Delcath” or the “Company”) an interventional oncology company focused on the treatment of primary and metastatic liver cancers, today announced the publication of a narrative review by leading interventional radiologists and oncologists from multiple institutions. The review, titled “Treatment of Liver Metastases from […]
Author: Ken Dropiewski
RapidAI Earns FDA Clearance for Rapid Aortic, Bringing AI-Driven Aortic Measurements and Surveillance to Care Teams
SAN MATEO, Calif.–(BUSINESS WIRE)–RapidAI, the pioneer of deep clinical AI and global leader in enterprise imaging, today announced U.S. Food and Drug Administration (FDA) clearance of Aortic Management, part of the Rapid Aortic product, a comprehensive deep clinical AI solution designed to transform the acute assessment and longitudinal management of aortic disease. […]
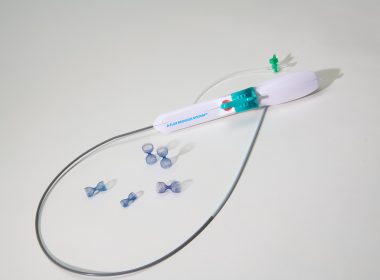
TriSalus Life Sciences Launches TriNav® XP Infusion System to Expand Options for Pressure-Enabled Drug Delivery™
WESTMINSTER, Colo.–(BUSINESS WIRE)–TriSalus Life Sciences, an oncology company integrating novel delivery technology with standard of care therapies to transform treatment for patients with solid tumors, is pleased to announce the launch of the TriNav® XP Infusion System, the latest advancement in the company’s portfolio of pressure-enabled drug delivery systems designed to improve […]
VenstraMedical Secures Investment From Highcroft Capital to Advance Next-generation Cardiac Support Device
NEWCASTLE, Australia & EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–VenstraMedical, a cardiovascular medical device company, today announced an investment from Highcroft Capital, a Minnesota-based venture capital firm specializing in MedTech innovation. The funding will accelerate development of VenstraMedical’s next-generation percutaneous ventricular assist device (pVAD) — a low-profile, full-flow system designed to provide complete […]
VahatiCor Closes Oversubscribed Series B Financing to Accelerate A-FLUX® Development for Coronary Microvascular Dysfunction.
The new capital will advance the SERRA-I early feasibility study, engineering development, and preparation for additional studies on coronary microvascular dysfunction. SANTA CLARA, Calif.–(BUSINESS WIRE)–VahatiCor, a medtech company transforming cardiac care for Coronary Microvascular Dysfunction (CMD), today announced the successful closing of its $23 million Series B financing led by S3 […]
CryoTherapeutics partners with SpectraWAVE and CRF to Launch ICECAP: A First-of-its-Kind Clinical Study Targeting Non-Obstructive High-Risk Coronary Plaques with Cryotherapy and Advanced Intravascular Imaging
LIÈGE, Belgium & BEDFORD, Mass.–(BUSINESS WIRE)–CryoTherapeutics, a medical device company developing minimally invasive localized cryotherapy for the treatment of coronary artery disease, today announced a unique three-way collaboration with SpectraWAVE, an innovator in intravascular imaging and physiology and the Cardiovascular Research Foundation (CRF), the global leader in cardiovascular research, to […]
Catheter Precision Announces Third VIVO Installation in France
First-ever multi-year agreement highlights commitment to French electrophysiology market First-ever multi-year agreement highlights commitment to French electrophysiology market
Nanox Announces Strategic Partnership with 3DR® Labs to Expand AI-Powered Imaging Solutions Across North America
3DR® Labs to Offer Nanox.AI’s FDA-Cleared Imaging Solutions to Its Network of More Than 1,800 Hospitals and Imaging Centers Across the U.S. PETACH TIKVA, Israel, Nov. 18, 2025 (GLOBE NEWSWIRE) — NANO-X IMAGING LTD (“Nanox” or the “Company”, Nasdaq: NNOX), an innovative medical imaging technology company, today announced a strategic reseller partnership with 3DR Labs, LLC, one of the largest providers of medical image post-processing services in the United States. Through this collaboration, 3DR Labs will offer Nanox.AI’s FDA-cleared solutions to its network of more than 1,800 hospitals, diagnostic imaging centers, and healthcare providers. Under the terms of the collaboration, 3DR Labs will integrate Nanox.AI’s advanced, FDA-cleared software solutions, HealthCCSng (AI cardiac solution), HealthOST (AI bone solution), and HealthFLD (AI liver solution), into its imaging services portfolio, making these AI-powered tools available to healthcare providers across its nationwide network. The partnership enables 3DR Labs to market, distribute Nanox.AI’s software solutions to its client base across North America. The agreement positions Nanox.AI’s technology to support early signs of disease detection and improve clinical outcomes management initiatives at scale across one of the largest medical imaging service networks in the United States. Founded in 2005, 3DR Labs provides continuous access to more than 300 expert radiologic technologists, cutting-edge imaging software, and AI-enabled workflow solutions. 3DR’s AI Labs, a vendor-agnostic platform, connects imaging departments to a gateway of 3D workflow automation, delivering quality, speed, and efficiency across healthcare systems. “This partnership represents one of our most significant U.S. reseller relationships to date and meaningfully expands our commercial reach,” said Erez Meltzer, CEO and Acting Chairman of Nanox. “3DR’s extensive, established relationships gives us a powerful channel to deliver our AI solutions to nearly two thousand healthcare organizations, which can use them to detect chronic diseases earlier.” Christy Mutchler, Vice President, Solutions Management of 3DR Labs, said, “Nanox.AI’s FDA-cleared solutions align perfectly with the population health priorities of the hospitals and imaging centers we serve. These tools can be seamlessly integrated into existing radiology workflows without disrupting clinical operations.” Nanox AI’s state-of-the-art software solutions use deep learning algorithms to automatically analyze routine CT scans for indicators of chronic disease that might otherwise go undetected: AI Cardiac solution, HealthCCSng, quantifies coronary artery calcium and classifies results according to Agatston categories, facilitating early detection of cardiovascular risk.AI Bone Solution, HealthOST, provides automated bone density and vertebral height loss evaluation for osteoporosis riskAI Liver Solution, HealthFLD, Liver attenuation measurement to identify patients at risk of fatty liver disease All three solutions are FDA-cleared and designed to integrate into existing radiology workflows, enabling opportunistic screening for multiple signs of chronic conditions from routine imaging studies. Nanox entered into a commercial agreement with 3DR, whose CEO also serves as a director at Nanox. The agreement was approved in accordance with the law applicable to related-party transactions. About Nanox.AI Nanox.AI is the deep-learning medical imaging analytics subsidiary of Nanox. Nanox.AI solutions are developed to target highly prevalent chronic and acute diseases affecting large populations around the world. Leveraging AI, Nanox.AI helps clinicians extract valuable and actionable clinical insights from medical imaging that otherwise may go unnoticed, potentially initiating further medical assessment to establish individual preventative care pathways for patients. For more information, please visit www.nanox.vision/ai. About Nanox Nanox (NASDAQ: NNOX) is focused on driving the world’s transition to preventive health care by bringing a full solution of affordable medical imaging technologies based on advanced AI and proprietary digital X-ray source. Nanox’s vision encompasses expanding the reach of Nanox technology both within and beyond hospital settings, providing a seamless end-to-end solution from scan to diagnosis, leveraging AI to enhance the efficiency of routine medical imaging technology and processes, in order to improve early detection and treatment and maintaining a clinically driven approach. The Nanox ecosystem includes Nanox.ARC – a multi-source digital tomosynthesis system that is cost-effective and user-friendly; Nanox.AI LTD – an AI-based suite of algorithms that augment the readings of routine CT imaging to highlight early signs often related to chronic diseases; Nanox.CLOUD – a cloud-based software platform that manages and stores data collected by Nanox devices, and provides users with tools for in-depth imaging analysis; Nanox.MARKETPLACE – a proprietary decentralized marketplace through Nanox’s subsidiary, USARAD Holdings Inc., that provides remote access to radiology and cardiology experts, and a comprehensive teleradiology services platform. By improving early detection and treatment, Nanox aims to enhance better health outcomes worldwide. For more information, please visit www.nanox.vision. About 3DR Labs + Strings 3DR® Labs and its wholly owned subsidiary, Strings, transform medical imaging and healthcare operations through advanced technology, intelligent automation, and trusted expertise. Headquartered in Louisville, Kentucky, 3DR Labs provides continuous access to more than 300 expert radiologic technologists, cutting-edge imaging software, and AI-enabled workflow solutions. 3DR’s AI Labs, a vendor-agnostic platform, connects imaging departments to a gateway of 3D workflow automation, delivering quality, speed, and efficiency across healthcare systems. Strings, a leader in AI-driven workflow orchestration, extends these capabilities by learning, predicting, and automating both human and data tasks. Purpose-built for healthcare, Strings empowers providers, IT teams, and administrators to streamline operations, reduce costs, and improve patient outcomes through predictive automation. Together, 3DR Labs and Strings deliver end-to-end innovation that enhances imaging operations, optimizes performance, drives smarter, faster, and better care delivery across the healthcare continuum. For more information, please visit 3drlabs.com. Forward-Looking Statements This press release may contain forward-looking statements that are subject to risks and uncertainties. All statements that are not historical facts contained in this press release are forward-looking statements. Such statements include, but are not limited to, any statements relating to the initiation, timing, progress and results of the Company’s research and development, manufacturing, and commercialization activities with respect to its X-ray source technology and the Nanox.ARC, the ability to realize the expected benefits of its recent acquisitions and the projected business prospects of the Company and the acquired companies. In some cases, you can identify forward-looking statements by terminology such as “can,” “might,” “believe,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “should,” “could,” “expect,” “predict,” “potential,” or the negative of these terms or other similar expressions. Forward-looking statements are based on information the Company has when those statements are made or management’s good faith belief as of that time with respect to future events and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Factors that could cause actual results to differ materially from those currently anticipated include: risks related to (i) Nanox’s ability to complete development of the Nanox System; (ii) Nanox’s ability to successfully demonstrate the feasibility of its technology for commercial applications; (iii) Nanox’s expectations regarding the necessity of, timing of filing for, and receipt and maintenance of, regulatory clearances or approvals regarding its technology, the Nanox.ARC and Nanox.CLOUD from regulatory agencies worldwide and its ongoing compliance with applicable quality standards and regulatory requirements; (iv) Nanox’s ability to realize the anticipated benefits of the acquisitions, which may be affected by, among other things, competition, brand recognition, the ability of the acquired companies to grow and manage growth profitably and retain their key employees; (v) Nanox’s ability to enter into and maintain commercially reasonable arrangements with third-party manufacturers and suppliers to manufacture the Nanox.ARC; (vi) the market acceptance of the Nanox System and the proposed pay-per-scan business model; (vii) Nanox’s expectations regarding collaborations with third-parties and their potential benefits; (viii) Nanox’s ability to conduct business globally; (ix) changes in global, political, economic, business, competitive, market and regulatory forces; (x) risks related to the current war between Israel and Hamas and any worsening of the situation in Israel; (xi) risks related to business interruptions resulting from the COVID-19 pandemic or similar public health crises, among other things; and (xii) potential litigation associated with our transactions. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Nanox’s actual results to differ from those contained in the Forward-Looking Statements, see the section titled “Risk Factors” in Nanox’s Annual Report on Form 20-F for the year ended December 31, 2024, and subsequent filings with the U.S. Securities and Exchange Commission. The reader should not place undue reliance on any forward-looking statements included in this press release. Except as required by law, Nanox undertakes no obligation to update publicly any forward-looking statements after the date of this press release to conform these statements to actual results or to changes in the Company’s expectations. Contacts Media Contact:Ben ShannonICR HealthcareNanoxPR@icrinc.com Elizabeth Morgan3DR Labsinfo@3drlabs.com Investor Contact:Mike CavanaughICR Healthcaremike.cavanaugh@icrhealthcare.com
Vasa Therapeutics Granted FDA Fast Track Designation for VS-041, a Novel Investigational Treatment of Heart Failure with Preserved Ejection Fraction (HFpEF)
Designation underscores the potential for VS-041 to address a significant unmet medical need and potentially improve diagnosis by utilizing a serum biomarker VS-041 is being evaluated in a Phase 1c study of participants with HFpEF and elevated serum endotrophin ENCINITAS, Calif., and…
Volta Medical and GE HealthCare Announce Successful U.S. Launch of Joint AI-Driven Electrophysiology Solutions
AF-Xplorer™ II is now fully integrated with GE HealthCare CardioLab™ AltiX Ai.i, aimed at delivering more efficient workflows and enhanced access to critical data MARSEILLE, France, Nov. 18, 2025 /PRNewswire/ — Volta Medical, committed to revolutionizing care for underserved complex AF…