Medtronic plc, a global leader in healthcare technology, today announced its Penditure™ Left Atrial Appendage (LAA) Exclusion System has officially received CE Mark approval and will be launched in Europe at the 39th European Association for Cardio-Thoracic Surgery (EACTS) Annual Meeting from October 8-11 in Copenhagen, Denmark. The Penditure device recently […]
Author: Ken Dropiewski
Positron Corporation Enters Industry Partnership Agreement with MedAxiom
Buffalo, NY, Oct. 02, 2025 (GLOBE NEWSWIRE) — Positron Corporation (“Positron” or the “Company”) (OTC: POSC), a leading molecular imaging technology company specializing in PET and PET-CT imaging systems and clinical services is pleased to announce a partnership with MedAxiom, the cardiovascular community’s premier source for organizational performance solutions.
LANDMARK PHASE 3 TRIAL (VESALIUS-CV) MEETS PRIMARY ENDPOINTS IN A CARDIOVASCULAR PRIMARY PREVENTION STUDY OF 12,000 PATIENTS
Adding Repatha to Standard Therapy of Statins or Other LDL-C Lowering Treatments Significantly Reduces Cardiovascular Events Compared with Standard Therapy Alone Repatha is Now the First and Only PCSK9 Inhibitor to Demonstrate Significant Reduction of Cardiovascular Events as Both Primary…
Groundbreaking Therapy for Advanced Heart Failure: Repairon Completes Series A Financing to Expand the Clinical Development of Its Regenerative Heart Therapy
GÖTTINGEN, Germany, Oct. 2, 2025 /PRNewswire/ — German biotech company Repairon GmbH announced the completion of a Series A round of financing that will enable further clinical development of its groundbreaking regenerative heart failure therapy. The therapy repairs cardiac function and…
AM-Pharma Completes Enrollment in Phase 2 Study of Ilofotase Alfa for Prevention of Cardiac Surgery-Associated Renal Damage
Utrecht, The Netherlands, October 2, 2025 – AM-Pharma B.V. today announced that it has successfully completed enrollment of patients in its ongoing Phase 2 clinical trial evaluating ilofotase alfa for the prevention of cardiac surgery-associated renal damage (CSA-RD). CSA-RD, a common cause of Acute Kidney Injury (AKI), is a serious complication of cardiac surgery occurring in up to 40% of patients. AKI following surgery is associated with both short- and long-term impairment of kidney function, an increased risk of requiring renal replacement therapy, sometimes lifelong, and higher mortality rates. Ilofotase alfa is the company’s proprietary therapeutic candidate that has consistently demonstrated safety, tolerability and reno-protective effects, including a reduction in Major Adverse Kidney Events (MAKE), across global clinical trials involving more than 1,000 patients diagnosed with AKI.
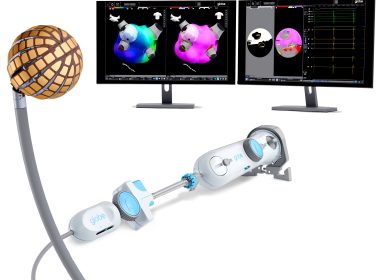
Kardium Announces First Commercial Procedures with the Globe® Pulsed Field System, the World’s Most Advanced Solution for Atrial Fibrillation
VANCOUVER, British Columbia–(BUSINESS WIRE)–Kardium, a private medical device company that’s advancing the way the world treats atrial fibrillation (AF), today announced the successful completion of the first commercial procedures using its integrated mapping and ablation system, the Globe Pulsed Field System, following recent approval from the U.S. Food and Drug Administration (FDA). The […]
Adagio Medical, Inc. Announces Completion of Enrollment for FULCRUM-VT Pivotal Clinical Trial Evaluating Ultra-Low Temperature Cryoablation for Ventricular Tachycardia
Study Results are Intended to Support PMA Approval of Adagio’s vCLASTM Cryoablation System Designed to Address the Large Population of Patients with Ventricular Arrhythmias LAGUNA HILLS, Calif.–(BUSINESS WIRE)–Adagio Medical Holdings, Inc. (Nasdaq: ADGM) (“Adagio” or “the Company”), a leading innovator in catheter ablation technologies for the treatment of cardiac arrhythmias, today […]
Switchback Medical Announces Strategic Investment From TJC and Combination With LightningCath and Proto Lase
NEW YORK–(BUSINESS WIRE)–Switchback Medical, LLC is pleased to announce a strategic investment from an affiliate of TJC, L.P. (“TJC”), a private investment firm. Switchback Medical, LLC has combined into a single platform with its affiliates LightningCath, Inc. and Proto Lase, Inc. (together, “Switchback” or the “Company”), with the businesses’ founders […]
Relief Cardiovascular Announces First-in-Human Procedures with World’s First Transcatheter Smart Implant for the Treatment of Congestion in Heart Failure
IRVINE, Calif., Sept. 30, 2025 /PRNewswire/ — Relief Cardiovascular, a private company developing transcatheter smart implants to transform the treatment of heart failure, today announced the successful first-in-human use of the Relief System – the world’s first implant designed to both hemodynamically monitor and treat congestion in heart failure. The breakthrough procedures were […]
Former Founding Director of the NIH Center for Interventional Oncology & Chief of Interventional Radiology, Dr. Bradford Wood, MD joins DeepSight as Vice President of Strategic Partnerships
SANTA CLARA, Calif., Oct. 1, 2025 /PRNewswire/ — DeepSight Technology, a leader in advanced interventional ultrasound solutions, is proud to announce the appointment of Bradford Wood, MD, formerly of the National Institutes of Health (NIH), as Vice President of Strategic Partnerships. Dr….