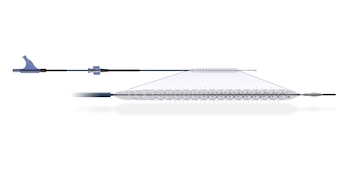
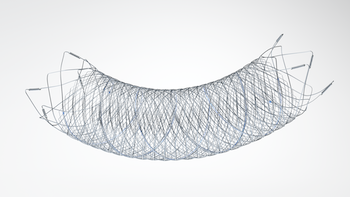
Positive pre-clinical and clinical proof-of-concept data of Sequana Medical’s innovative DSR therapy published in top tier peer-reviewed cardiovascular journal Data demonstrates potential for treatment of volume overload due to heart failure Ghent, BELGIUM – 10 January 2020 – Sequana Medical NV (Euronext Brussels: SEQUA), an innovator in the management of fluid […]
Author: Ken Dropiewski
Akcea and Ionis Announce Initiation of CARDIO-TTRansform Phase 3 Clinical Trial for AKCEA-TTR-LRx in Patients with TTR-mediated Amyloid Cardiomyopathy
BOSTON and CARLSBAD, Calif., Jan. 10, 2020 (GLOBE NEWSWIRE) — Akcea Therapeutics, Inc. (NASDAQ: AKCA), a majority-owned affiliate of Ionis Pharmaceuticals, Inc., and Ionis Pharmaceuticals, Inc. (NASDAQ: IONS), announced today the initiation of the CARDIO-TTRansform Phase 3 cardiovascular outcomes study for AKCEA-TTR-LRx in patients with transthyretin-mediated amyloid cardiomyopathy (ATTR cardiomyopathy). “The […]
Matinas BioPharma Announces Proposed Public Offering of Common Stock
BEDMINSTER, N.J., Jan. 09, 2020 (GLOBE NEWSWIRE) — Matinas BioPharma Holdings, Inc. (“Matinas BioPharma” or the “Company”) — (NYSE AMER: MTNB), a clinical-stage biopharmaceutical company, today announced that it has commenced an underwritten public offering of its common stock. In connection with the offering, Matinas BioPharma intends to grant the […]
Cardiva Medical Appoints Jeri Hilleman to the Company’s Board of Directors
SANTA CLARA, Calif.–(BUSINESS WIRE)–Cardiva Medical, Inc., an innovator in the field of vascular closure, today announced the appointment of medical device expert Jeryl (“Jeri”) Hilleman to the company’s Board of Directors. “We are thrilled to welcome Jeri to our team and look forward to leveraging her deep expertise with high-growth […]
FDA Grants Breakthrough Device Designation to Reflow Medical’s Temporary Spur Stent System
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical announces that the Temporary Spur Stent System, a novel retrievable stent technology intended for the treatment of below-the-knee (BTK) peripheral artery disease, has been designated for the Breakthrough Devices Program by the U.S. Food and Drug Administration (FDA). The Breakthrough Devices Program is designed to […]
JenaValve Transcatheter Aortic Valve Replacement (TAVR) System Designated by FDA as Breakthrough Device
JenaValve Pericardial TAVR System is the First Transcatheter Device to Achieve Breakthrough Device Designation; Prioritizes Premarket Approval Development Path for Severe Aortic Regurgitation Indication IRVINE, Calif.–(BUSINESS WIRE)–JenaValve Technology, Inc., developer and manufacturer of the JenaValve Pericardial transcatheter aortic valve replacement (TAVR) system for the treatment of aortic valve disease, announced […]
FAST BioMedical Announces Successful Completion of Multi-center Acute Congestive Heart Failure Trial in Germany
INDIANAPOLIS and BERLIN and BAD NAUHEIM, Germany, Jan. 9, 2020 /PRNewswire/ — FAST BioMedical announced the completion of the EMPAKT-CHF clinical trial at Charité – Universitätsmedizin Berlin and Kerckhoff Klinik in Bad Nauheim. The trial evaluated the impact of FAST BioMedical’s Blood Volume and Kidney Function measurement technology in patients with acute congestive heart failure and cardiorenal syndrome. This […]
Alucent Biomedical Announces FDA Approval to Proceed with Natural Vascular Scaffolding Clinical Trial
SALT LAKE CITY–(BUSINESS WIRE)–Alucent Biomedical Inc. has received U.S. Food and Drug Administration approval to proceed with a Phase 1 clinical trial to evaluate the safety and efficacy of its revolutionary Natural Vascular Scaffolding (NVS) technology. The therapy is designed to treat peripheral artery disease (PAD) of the lower extremities, a […]
Penumbra Announces FDA Clearance of Indigo® Aspiration System for Treatment of Pulmonary Embolism
ALAMEDA, Calif.–(BUSINESS WIRE)–Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, today announced U.S. Food and Drug Administration 510(k) clearance for expanded indication of the Indigo® Aspiration System. As part of the Indigo Aspiration System, the Indigo Aspiration Catheters and Separators are indicated for the removal of fresh, […]
MicroVention Announces FDA Premarket Approval of a New Flow Diverter for the Treatment of Brain Aneurysms
Aliso Viejo, CA. – January 9, 2020 –MicroVention, Inc., a U.S. based subsidiary of Terumo and a global neurovascular company announced the FDA Premarket Approval (PMA) for the FRED® (Flow Re-Direction Endoluminal Device) device for the treatment of brain aneurysms. The FRED® device is the first flow diverter in the U.S. to use […]