NASDAQ: CORV TSX: CORV VANCOUVER, Dec. 24, 2019 /PRNewswire/ – Correvio Pharma Corp. (NASDAQ: CORV) (TSX: CORV), a specialty pharmaceutical company focused on commercializing hospital drugs, today announced it has received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) regarding the New Drug Application (NDA) for Brinavess™ (vernakalant IV), an anti-arrhythmic […]
Author: Ken Dropiewski
Acasti Pharma Provides Update on Timing of Topline Results for TRILOGY 1 Phase 3 Trial of CaPre
Expects to report Trilogy 1 topline results in January 2020 with topline results for Trilogy 2 still expected by the end of January 2020 LAVAL, Québec, Dec. 23, 2019 (GLOBE NEWSWIRE) — Acasti Pharma Inc. (“Acasti or the “Company”) (NASDAQ: ACST – TSX-V: ACST), a biopharmaceutical innovator focused on the […]
Endonovo Therapeutics Announces Effective Date of Previously Announced Reverse Split of Common Stock
Los Angeles, CA, Dec. 19, 2019 (GLOBE NEWSWIRE) — Endonovo Therapeutics, Inc. (OTCQB: ENDV) (“Endonovo” or the “Company”), announced today that its previously announced 1-for-1,000 reverse split of its common stock will become effective as of December 19, 2019. Beginning on December 20, 2019, the Company’s common stock will trade […]
Resverlogix Appoints Dicky To to its Board of Directors
CALGARY, Alberta, Dec. 20, 2019 (GLOBE NEWSWIRE) — Resverlogix Corp. (“Resverlogix” or the “Company”) (TSX: RVX) today announced that Mr. Dicky To has been appointed to the Company’s Board of Directors. His appointment is effective as of December 19, 2019. Dicky is a partner of ORI Capital and has over […]
CMS Approves Coverage for PQ Bypass TORUS 2 IDE Trial
Latest accomplishment reached by Silicon Valley med-tech company currently enrolling two IDE studies MILPITAS, Calif.–(BUSINESS WIRE)–PQ Bypass Inc, a medical device company bringing new advancements to the treatment of peripheral artery disease (PAD), announced today that it has received approval for coverage from the Centers for Medicare and Medicaid Services […]
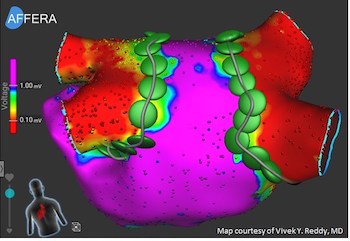
Affera Announces World’s First Successful Focal Pulsed Field Ablation in Patients
WATERTOWN, Mass., Dec. 19, 2019 /PRNewswire/ — Affera, Inc., a private medical device company focused on innovative cardiac arrhythmia treatment solutions, announced today that its focal Pulsed Field (“PF”) ablation technology, also known as Irreversible Electroporation (“IRE”), has been successfully used to treat 40 patients suffering from Atrial Fibrillation (“AFIB”). This multi-center […]
Quantum Genomics Initiates its Pivotal Phase III FRESH Trial in Difficult-to-Treat and Resistant Hypertension
PARIS and NEW YORK, Dec. 19, 2019 (GLOBE NEWSWIRE) — Quantum Genomics (Euronext Growth – FR0011648971 – ALQGC, OTCQX – QNNTF), a biopharmaceutical company specializing in developing a new drug class that directly targets the brain to treat resistant hypertension and heart failure, today announced the initiation of its pivotal Phase III FRESH study […]
CryoLife and Misonix Enter Into Distribution Agreement for NeoPatch
CryoLife Will Supply NeoPatch, a Chorioamniotic Membrane Tissue, to Misonix ATLANTA, Dec. 19, 2019 /PRNewswire/ — CryoLife, Inc. (“CryoLife”;NYSE: CRY), a leading cardiac and vascular surgery company focused on aortic disease, announced today that it has entered into an agreement whereby Misonix (NASDAQ: MSON) will have exclusive US commercialization rights for CryoLife’s NeoPatch product to […]
Results Demonstrating Favorable Outcomes with TCAR vs. TF-CAS in Patients with Carotid Artery Stenosis Published in the Journal of the American Medical Association (JAMA)
SUNNYVALE, Calif., Dec. 17, 2019 (GLOBE NEWSWIRE) — Silk Road Medical, Inc. (Nasdaq: SILK), a company focused on reducing the risk of stroke and its devastating impact, today announced that positive results from the ongoing TransCarotid Artery Revascularization (TCAR) Surveillance Project comparing TCAR and transfemoral carotid artery stenting (TF-CAS) have been published in The Journal […]
FDA Grants ‘Breakthrough’ Designation to Eko’s ECG-based Low Ejection Fraction Screening Algorithm, Designed to Improve Detection of Heart Failure
Developed in collaboration with Mayo Clinic, the algorithm would help healthcare providers detect heart failure during a standard physical exam San Francisco, CA, Dec. 18, 2019 (GLOBE NEWSWIRE) — Eko, a digital health company applying artificial intelligence (AI) in the fight against heart disease, today announced the U.S. Food and Drug Administration […]