MONTREAL, Nov. 5, 2025 /PRNewswire/ – Thryv Therapeutics Inc., a clinical-stage biotechnology company advancing a series of novel serum glucocorticoid inducible kinase 1 (SGK1) inhibitors for inherited cardiac arrhythmias, cardiometabolic diseases, and cardiomyopathies, today announced…
Coronary/Structural Heart
NCH Becomes First U.S. Hospital to Use AI That Detects Hidden Heart Attack Risk a Decade in Advance
Landmark collaboration with Caristo Diagnostics positions NCH at the forefront of preventive cardiology NCH cardiologists to present collaboration plans at American Heart Association 2025 Scientific Sessions on Nov. 8 NAPLES, Fla. and STAMFORD, Conn. , Nov. 5, 2025 /PRNewswire/ — Naples…
Midwest Cardiovascular Institute and Basata Partner to Modernize Cardiac Care with Voice AI
Partnership introduces AI-powered voice technology to improve patient access and reduce wait times. CHICAGO, Nov. 5, 2025 /PRNewswire/ — Midwest Cardiovascular Institute (MCI), a leading provider of cardiovascular care in Chicagoland, today announced a partnership with Basata, a…
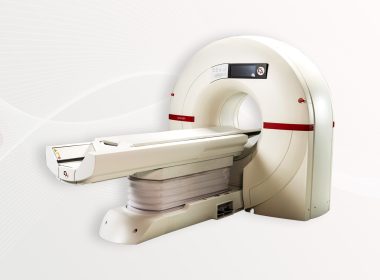
Dallas Cardiology Group Expands Access to Advanced Cardiac Imaging in North Texas Following High Patient Demand
Connected Cardiovascular Care Associates brings Arineta’s SpotLight scanner to its Rockwall location, broadening availability to the same technology they first brought to Dallas in 2023 Dallas, TX – October 29, 2025 – Connected Cardiovascular Care Associates (C3), a Dallas-based cardiology practice, is expanding its Arineta SpotLight installations that provide […]
Tulyp Medical Emerges From Sofinnova’s Medtech Accelerator and Announces FDA Submission Following Initial First-in-Human Use
Company unveils patented technology designed to help maintain healthy blood flow during vascular procedures Backed by Sofinnova MD Start and led by experienced medtech entrepreneurs and cardiologists PARIS–(BUSINESS WIRE)–Tulyp Medical, a Paris-based startup developing a medical device for intelligent pressure-driven perfusion, emerged from stealth and announced it has submitted its […]
Data Demonstrating Unique Benefits of Sotagliflozin in Diverse Patient Populations will be Presented at the Hypertrophic Cardiomyopathy Society and American Heart Association’s Sessions 2025 in New Orleans
Study results further differentiate sotagliflozin from SGLT inhibitors and cardiac myosin inhibitorsTHE WOODLANDS, Texas, Nov. 04, 2025 (GLOBE NEWSWIRE) — Lexicon Pharmaceuticals, Inc. (Nasdaq: LXRX) today announced that data from three studies of sotagliflozin will be presented during upcoming medical meetings. Two studies will be presented at the American Heart Association’s (AHA) Scientific Sessions 2025, being held November 7-10 at the Ernest N. Morial Convention Center in New Orleans, Louisiana, and online, while the third study will be presented at the Hypertrophic Cardiomyopathy Society (HCMS) Scientific Sessions, which this year is conducting its conference at AHA’s Scientific Sessions. “We believe that these presentations will further position sotagliflozin, a dual SGLT 1 and 2 inhibitior, as a class of medication different from any other on the market,” said Craig Granowitz, M.D., Ph.D., Lexicon’s senior vice president and chief medical officer. “As additional data is presented at December’s CVCT 2025 and beyond, we expect to build on the compelling findings being presented at HCMS and AHA.” Details of the three presentations are as follows: Dual SGLT1 and 2 Inhibition with Sotagliflozin Ameliorates Adverse Cardiac Remodeling and Diastolic Dysfunction in Mice with HCM Due to Tropomyosin E180E Mutation — an oral presentation Friday, November 7th, 3:00 – 3:10 p.m. CT, New Orleans Ernest N. Morial Convention Center R02-R03, presented by Fuzhong Qin, M.D., Ph.D., Professor, Boston University Chobanian and Avedisian School of Medicine, Boston, MA.SOTA-P-CARDIA: A Randomized Trial of Sotagliflozin in HFpEF patients without diabetes — an oral presentation at a Late-breaking Science Session on Saturday, November 8th, 2:20 – 2:28 p.m. CT, New Orleans Ernest N. Morial Convention Center Room 211-213, presented by Juan Jose Badimon, Ph.D., Professor of Medicine and Director of the Atherothrombosis Research Unit at the Cardiovascular Institute, The Mount Sinai School of Medicine, New York, NY.Effects of major adverse cardiovascular events in persons treated with sotagliflozin: Prespecified pooled analyses of the Phase 3 type 2 diabetes program — a poster presentation Saturday, November 8th, 2:30 – 3:30p.m. CT, New Orleans Ernest N. Morial Convention Center Clinical Science Zone #2, presented by Darren K. McGuire, M.D., M.H.Sc., Professor of Internal Medicine, Division of Cardiology, UT Southwestern Medical Center, Dallas, TX. About SotagliflozinDiscovered using Lexicon’s unique approach to gene science, sotagliflozin is an oral inhibitor of two proteins responsible for glucose regulation known as sodium-glucose cotransporter types 2 and 1 (SGLT2 and SGLT1). SGLT2 is responsible for glucose and sodium reabsorption by the kidney and SGLT1 is responsible for glucose and sodium absorption in the gastrointestinal tract. Sotagliflozin has been studied in multiple patient populations encompassing heart failure, diabetes, and chronic kidney disease in clinical studies involving approximately 20,000 patients. Sotagliflozin is also currently under investigation for another cardiac condition, hypertrophic cardiomyopathy (HCM).About Lexicon Pharmaceuticals Lexicon is a biopharmaceutical company with a mission of pioneering medicines that transform patients’ lives. Through the Genome5000™ program, Lexicon’s unique genomics target discovery platform, Lexicon scientists studied the role and function of nearly 5,000 genes and identified more than 100 protein targets with significant therapeutic potential in a range of diseases. Through the precise targeting of these proteins, Lexicon is pioneering the discovery and development of innovative medicines to treat disease safely and effectively. Lexicon has a pipeline of promising drug candidates in discovery and clinical and preclinical development in neuropathic pain, HCM, obesity, metabolism and other indications. For additional information, please visit www.lexpharma.com. Safe Harbor Statement This press release contains “forward-looking statements,” including statements relating to Lexicon’s financial position and long-term outlook on its business, including the commercialization of its approved products and the clinical development of, regulatory filings for, and potential therapeutic and commercial potential of sotagliflozin and its other drug candidates. In addition, this press release also contains forward looking statements relating to Lexicon’s growth and future operating results, discovery, development and commercialization of products, strategic alliances and intellectual property, as well as other matters that are not historical facts or information. All forward-looking statements are based on management’s current assumptions and expectations and involve risks, uncertainties and other important factors, specifically including Lexicon’s ability to meet its capital requirements, successfully commercialize its approved products, successfully conduct preclinical and clinical development and obtain necessary regulatory approvals of its other drug candidates on its anticipated timelines, achieve its operational objectives, obtain patent protection for its discoveries and establish strategic alliances, as well as additional factors relating to manufacturing, intellectual property rights, and the therapeutic or commercial value of its approved products and other drug candidates. Any of these risks, uncertainties and other factors may cause Lexicon’s actual results to be materially different from any future results expressed or implied by such forward-looking statements. Information identifying such important factors is contained under “Risk Factors” in Lexicon’s annual report on Form 10-K for the year ended December 31, 2024, as filed with the Securities and Exchange Commission. Lexicon undertakes no obligation to update or revise any such forward-looking statements, whether as a result of new information, future events or otherwise. For Investor and Media Inquiries: Lisa DeFrancesco Lexicon Pharmaceuticals, Inc. lexinvest@lexpharma.com
Kestra Medical Technologies to Present Late-Breaking ACE-PAS Trial Results at AHA 2025, Showcasing Next-Generation Wearable Defibrillator System
KIRKLAND, Wash., Nov. 04, 2025 (GLOBE NEWSWIRE) — Kestra Medical Technologies, Ltd. (Nasdaq: KMTS), a wearable medical device and digital healthcare company, today announced its participation in the American Heart Association (AHA) Scientific Sessions 2025, to be held November 7–10 in New Orleans. Kestra’s participation will include a late-breaking science presentation titled Primary Results from the Post-Approval Study of a Next Generation Wearable Cardioverter Defibrillator System (ACE-PAS Trial), scheduled for Monday, November 10 at 8:44 a.m. CT in Session 211–213. The ASSURE® WCD Clinical Evaluation Post-Approval Study (ACE-PAS) is a contemporary study designed to evaluate real-world experience with a next-generation wearable cardioverter defibrillator system. Enrolling more than 20,000 patients across the U.S., ACE-PAS represents the largest prospective WCD study conducted to date. Primary endpoints include overall shock conversion success and inappropriate shock rate, with additional measures evaluating first shock conversion success, median daily wear time, and false alarm rate. The study’s selection for AHA’s late-breaking science program underscores how wearable defibrillator therapy, and data-driven insights, are advancing the way clinicians approach cardiac recovery and risk protection. “Being selected for a late-breaking presentation at AHA reflects both the clinical importance of the ACE-PAS study and the growing recognition of Kestra’s next-generation technology,” said Brian Webster, President and Chief Executive Officer of Kestra Medical Technologies. “As the cardiac recovery landscape evolves, we’re redefining how wearable monitoring and therapy integrates with connected care and digital health innovation—advancing patient protection and delivering deeper clinical insight.” At booth #4617, attendees can explore the Kestra Cardiac Recovery System®, anchored by the ASSURE® Wearable Cardioverter Defibrillator, uniting proven patient protection with the clinical insights providers need to optimize guideline-directed medical therapy, implantable cardioverter defibrillator evaluation, and long-term recovery. In addition to the late-breaking presentation, Kestra will host live demonstrations and discussions throughout the meeting, featuring an immersive in-booth experience that highlights the company’s leadership in advancing connected, data-driven solutions for cardiac recovery. About Kestra Kestra Medical Technologies, Ltd. is a commercial-stage wearable medical device and digital healthcare company focused on transforming patient outcomes in cardiovascular disease using monitoring and therapeutic intervention technologies that are intuitive, intelligent, and connected. For more information, please visit www.kestramedical.com. CONTACT: Media contact
Rhiannon Pickus
rhiannon.pickus@kestramedical.com
Investor contact
Neil Bhalodkar
neil.bhalodkar@kestramedical.com
Bitterroot Bio to Present Anti-CD47 Research at American Heart Association Annual Scientific Session 2025
PALO ALTO, Calif. and NEEDHAM, Mass., Nov. 03, 2025 (GLOBE NEWSWIRE) — Bitterroot Bio, a leader in developing innovative medicines in the field of cardio-immunology, announced today that the Company will present data from its anti-CD47 research at the American Heart Association (AHA) Annual Scientific Session 2025, taking place in New Orleans, Louisiana, November 7 – 10, 2025. AHA Abstracts Cardioprotection in a Rat Model of Ischemic Injury by a Novel Anti-CD47 Fusion Protein Presentation: Sa3080 (Heart Failure, Cardiomyopathy & Pulmonary Hypertension)Time: Saturday, November 8th at 10:30am CTPresenting Author: Pierre E. Signore, PhD Anti-CD47 Therapy Induces Early Macrophage-Specific Transcriptomic Changes Associated with the Resolution of Inflammation in a Murine Model of Atherosclerosis Presentation: Su4023 (Genetics and Genomics)Time: Sunday, November 9th at 11:30am CTPresenting Author: Derek Klarin, MD The posters will be available on the Bitterroot Bio website after the presentations. About Bitterroot BioBitterroot Bio, Inc. is a pioneer in the field of cardio-immunology, which investigates the interplay between the immune system and cardiovascular health. Bitterroot Bio’s research seeks to uncover critical roles that immune modulators play in the progression of cardiovascular disease. By targeting these diseases in this novel way, Bitterroot Bio’s mission is to transform the lives of patients suffering from cardiovascular diseases. For more information, please visit https://www.brbio.com or follow us on Facebook LinkedIn, or X. Media Contact: Pablo Fenton (Bitterroot Bio), media@brbio.com
Heartflow to Present Late-Breaking Data Advancing AI-Driven Heartflow Plaque Analysis at AHA 2025
Data to highlight the precision of AI-powered Heartflow Plaque Staging framework in predicting long-term cardiovascular outcomesMOUNTAIN VIEW, Calif., Nov. 03, 2025 (GLOBE NEWSWIRE) — Heartflow, Inc. (Heartflow) (Nasdaq: HTFL), the leader in AI technology for coronary artery disease (CAD), today announced it will present new late-breaking data highlighting the clinical and economic value of Heartflow Plaque Analysis at the American Heart Association (AHA) Scientific Sessions 2025, taking place November 7-10 in New Orleans. The late-breaking Plaque Analysis data evaluate risk prediction with Heartflow Plaque Staging* and will remain under embargo until the featured science presentation, “Coronary CT Angiography Plaque as a Predictor of Death, Cardiovascular Death and Myocardial Infarction” on Sunday, November 9, at 12:30 p.m. CST. Timothy Fairbairn, M.D., principal investigator for the FISH&CHIPS study, Liverpool Heart and Chest Hospital NHS Foundation Trust, and Associate Professor at the University of Liverpool, will present the study. With seven abstracts at this year’s AHA Scientific Sessions, Heartflow is significantly advancing the scientific foundation for Plaque Analysis as a key advance guiding cardiovascular risk prediction and personalized treatment. As the established leader in AI-driven coronary care, Heartflow continues to invest in high-quality research to benefit all stakeholders in the healthcare system, fully demonstrating the utility of the Heartflow One platform in transforming CAD into a manageable disease. “Heartflow’s presentations at the AHA Scientific Sessions reflect our ongoing investment in clinical research to validate Plaque Analysis with Heartflow Plaque Staging, empowering cardiologists to personalize treatment plans using non-invasive precision technology,” said Campbell Rogers, M.D., F.A.C.C., Chief Medical Officer at Heartflow. “By providing AI-driven insights into plaque quantification, we’re helping clinicians see the full picture of coronary artery disease and building more effective pathways for diagnosis, management, and prevention.” Heartflow’s data presentations at AHA will include: Coronary CT Angiography Plaque as a Predictor of Death, Cardiovascular Death and Myocardial Infarction Session: Pan Vascular Interventions: Anatomy and Interventions Across Various Vascular BedsPresenter: Timothy Fairbairn, M.D.Date: Sunday, November 9Time: 12:30-12:38 p.m. CSTLocation: Clinical Science Zone 1‚ Moderated Digital Poster 5 Improvements in Diagnostic and Therapeutic Cardiovascular Risk Assessment Through Total Plaque Volume Burden: An Analysis of the FISH&CHIPS StudyFinalist for the Quest Diagnostics Early Career Investigator Award for Preventive Cardiovascular Medicine Research Session: Quest Diagnostics Early Career Investigator Award for Preventive Cardiovascular Medicine Research CompetitionPresenter: Shyon Parsa, M.D., Internal Medicine Resident at Stanford University HospitalDate: Sunday, November 9Time: 10:30-10:40 a.m. CSTLocation: 208-209 Population Changes in AI Coronary Plaque Volumes over 7 Years Session: New Frontiers in Cardiovascular Risk: Trends and Drivers in Cardiovascular Mortality and OutcomesPresenters: Allen Taylor, M.D., F.A.C.C., F.A.H.A., Chairman of Cardiology, MedStar Heart and Vascular Institute and Professor of Medicine at Georgetown University and Matthew Budoff, M.D., Professor of Medicine at the David Geffen School of Medicine at the University of California Los Angeles (UCLA) Medical CenterDate: Saturday, November 8Time: 10:30-11:30 a.m. CSTLocation: Population Science Zone AI-enabled Plaque Phenotype Analysis of Coronary Computed Tomography Angiography Findings in Patients with Nonacute Chest Pain using FFRCT: Results from the PRECISE Trial Session: Imaging Insights from Multicenter Clinical TrialsPresenter: Jonathon Leipsic, M.D., F.R.C.P.C., M.S.C.C.T., Professor and Chair of Radiology and Professor of Cardiology at the University of British ColumbiaDate: Saturday, November 8Time: 11:34-11:39 a.m. CSTLocation: Clinical Science Zone 1‚ Moderated Digital Poster 8 Cost-Effectiveness of AI-Enabled Coronary Plaque Analysis for Management of Stable Coronary Artery Disease Session: Transforming Cardiac Imaging and Risk Assessment Through AIPresenter: Daniel D’Attilio, Director, Healthcare Economics and Outcomes Research, HeartflowDate: Saturday, November 8Time: 12:36-12:41 p.m. CSTLocation: Clinical Science Zone 1‚ Moderated Digital Poster 4 Statin Use Mitigates Androgen Deprivation Therapy-Associated Coronary Atherosclerosis in Prostate Cancer: A Secondary Analysis of the REVELUTION Randomized Clinical Trial Session: Cardiac Amyloidosis and Cardiometabolic ConundrumsPresenter: Marly van Assen, Ph.D., Co-director of the Translational Laboratory for Cardiothoracic Imaging and Artificial Intelligence, Head of CT and AI Research, and Assistant Professor, Emory UniversityDate: Saturday, November 8Time: 2:27-2:32 p.m. CSTLocation: Clinical Science Zone 2‚ Moderated Digital Poster 19 Accelerated Coronary Atherosclerosis Following Relugolix Versus Leuprolide Androgen Deprivation Therapy in Men with Prostate Cancer (REVELUTION): An Open-Label Randomized Controlled Trial Session: Where Cancer and Cardiovascular Disease Collide: Risks, Disparities, and Evolving EvidencePresenter: Marly van Assen, Ph.D., Co-director of the Translational Laboratory for Cardiothoracic Imaging and Artificial Intelligence, Head of CT and AI Research, and Assistant Professor, Emory UniversityDate: Saturday, November 8Time: 3:15-3:20 p.m. CSTLocation: Population Science Zone‚ Moderated Digital Poster 12 Heartflow invites AHA attendees to a Heart Theater Symposium exploring how AI-driven plaque quantification is transforming CAD management: AI-Plaque Analysis: The Paradigm Shift in CAD Management Moderator: Seth Martin, M.D., M.H.S., FAHA, Professor of Medicine and Cardiologist at Johns Hopkins School of MedicinePanelists: Fatima Rodriguez, M.D., F.A.C.C., Section Chief of Preventive Cardiology and Associate Professor in the Division of Cardiovascular Medicine at Stanford University, and Ron Blankstein, M.D., Director of Cardiac Computed Tomography at Brigham and Women’s Hospital and Professor of Medicine at Harvard Medical SchoolDate: Sunday, November 9Time: 3:15-4:00 p.m. CSTLocation: Heart Theater 1 *Heartflow Plaque Analysis is an FDA-cleared device. Heartflow Plaque Staging is an investigational-only framework and its safety and effectiveness have not been reviewed by the FDA. About Heartflow’s Technology and ResearchHeartflow’s technology is redefining precision cardiovascular care through clinically-proven AI and the world’s largest coronary imaging dataset. Heartflow has been adopted by more than 1,400 institutions globally and continues to strengthen its commercial presence to make this cutting-edge solution more widely available to an increasingly diverse patient population. Backed by ACC/AHA guidelines and supported by more than 600 peer-reviewed publications, Heartflow has redefined how clinicians manage care for nearly 500,000 patients worldwide. Key benefits include: Proprietary data pipeline: Built from more than 110 million annotated CTA images, Heartflow’s data foundation powers advanced AI models that deliver highly accurate, reproducible insights across diverse patient populations.Extensive clinical and real-world validation: Heartflow’s AI-driven solutions have been validated through clinical evidence in over 100 studies assessing over 365,000 patients. Proven in real-world practice with reproducibility and accuracy, Heartflow’s coronary CTA image acceptance rates exceed 96%.Seamless clinical integration via upgraded workflow: Heartflow delivers final quality-reviewed analyses instantly upon order, enabling clinicians to move from diagnosis to decision without delay.Quality system, global security and patient-data integrity compliance: Heartflow meets or exceeds leading international standards, including HITRUST, SOC 2 Type 2, GDPR, HIPAA, CCPA, ISO 13485, and ISO 27001. About Heartflow, Inc.Heartflow is transforming coronary artery disease from the world’s leading cause of death into a condition that can be detected early, diagnosed accurately, and managed for life. The Heartflow One platform uses AI to turn coronary CTA images into personalized 3D models of the heart, providing clinically meaningful, actionable insights into plaque location, volume, and composition and its effect on blood flow — all without invasive procedures. Discover how we’re shaping the future of cardiovascular care at heartflow.com. Media ContactElliot Levyelevy@heartflow.com Investor ContactNick Laudiconlaudico@heartflow.com
Picard to Showcase its Total Artificial Heart at the Annual Meeting of the American Heart Association 2025
TUCSON, Ariz., Nov. 03, 2025 (GLOBE NEWSWIRE) — Picard Medical, Inc. (NYSE American: PMI) (“Picard” or the “Company”), parent company of SynCardia Systems LLC, maker of the world’s first U.S. and Canadian commercially-approved total artificial heart, today announced it will exhibit at the annual meeting of the American Heart Association (AHA) being held November 7th through 10th in New Orleans, Louisiana. At the annual meeting of the AHA, Picard will showcase its SynCardia™ Total Artificial Heart (or “STAH”), which is the most widely used and extensively studied artificial heart in the world, at booth #3408. Patrick NJ Schnegelsberg, Chief Executive Officer of Picard Medical, Inc. commented, “We are excited to attend the annual meeting of the American Heart Association to share the latest developments related to our innovative and life-saving artificial heart, the Syncardia Total Artificial Heart, or STAH. We look forward to having discussions with physicians and attendees at AHA about patient eligibility, the latest clinical data and helpful resources, and sharing insights from patients who have had real-world experiences with the STAH.” About the American Heart Association (AHA) and its Annual Meeting #AHA25 brings together the full spectrum of cardiovascular science—from foundational science and translational breakthroughs to clinical trials and real-world application. Each year, thousands of professionals come together to exchange ideas, build lasting collaborations, and accelerate progress in cardiovascular health on a global scale. The AHA has grown into the nation’s oldest and largest voluntary organization dedicated to fighting heart disease and stroke. A shared focus on cardiovascular health unites its more than 35 million volunteers and supporters as well as its more than 3,300 employees. Learn more here. The AHA has invested more than $6 billion in research, making it the largest not-for-profit funding source for cardiovascular and cerebrovascular disease research next to the federal government. About Picard Medical and SynCardia Picard Medical, Inc. is the parent company of SynCardia Systems, LLC (“SynCardia”), the Tucson, Arizona–based leader with the only commercially available total artificial heart technology for patients with end-stage heart failure. SynCardia develops, manufactures, and commercializes the SynCardia Total Artificial Heart (“STAH”), an implantable system that assumes the full functions of a failing or failed human heart. It is the first artificial heart approved by both the FDA and Health Canada, and it remains the only commercially available artificial heart in the United States and Canada. With more than 2,100 implants performed at hospitals across 27 countries, the SynCardia Total Artificial Heart is the most widely used and extensively studied artificial heart in the world. For additional information about Picard Medical, please visit www.picardmedical.com or review the Company’s filings with the U.S. Securities and Exchange Commission at www.sec.gov. Forward-Looking Statements This release may contain forward-looking statements within the meaning of federal securities laws. Such statements are based on current expectations and are subject to risks and uncertainties that may cause actual results to differ materially. The Company undertakes no obligation to update or revise any forward-looking statements. Contact: InvestorsEric RibnerManaging DirectorLifeSci Advisors LLCeric@lifesciadvisors.com Picard Medical, Inc./SynCardia Systems, LLCIR@picardmedical.com General/MediaBrittany Lanzablanza@syncardia.com