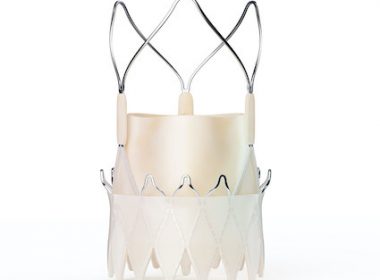
London Valves 2022: Latest Transcatheter Aortic Valve Replacement Data for Treatment of Aortic Stenosis and Prosthetic Aortic Valve Degeneration Medtronic’s newest generation self-expanding, transcatheter aortic valve replacement (TAVR) system, Evolut™ FX, significantly improved commissure alignment during TAVR procedures compared to earlier generation Evolut systems. A late breaker presentation at PCR […]
Coronary/Structural Heart
UAB Cardiogenomics Clinic and Allelica to perform polygenic risk score clinical implementation study
The Cardiogenomics Clinic at the University of Alabama at Birmingham and Allelica are partnering to perform a clinical implementation study to assess how polygenic risk score integration changes clinical management and health outcomes in a US health system. NEW YORK, Nov. 29, 2022 /PRNewswire/ — The UAB Cardiogenomics Clinic, led by Dr. Pankaj Arora, is […]
Neovasc Announces Progress on COSIRA-II Clinical Trial
VANCOUVER and MINNEAPOLIS, Nov. 28, 2022 (GLOBE NEWSWIRE) — via NewMediaWire – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ, TSX: NVCN) today announced an update on the COSIRA-II Clinical Trial (“Trial”) status. COSIRA-II is the U.S. Investigational Device Exemption (“IDE”) pivotal clinical trial the Company is executing for the Neovasc Reducer™ (“Reducer”) towards an approval […]
FEops’ AI-enabled Solution Changes TAVI Pre-procedural Decision in One Third of Patients, Associated With Favorable Clinical Outcomes
GENT, Belgium–(BUSINESS WIRE)–Data from the PRECISE-TAVI study, presented at the PCR Valves 2022 convention in London shows that Transcatheter Aortic Valve Implantation (TAVI) procedures planned by means of FEops HEARTguideTM result in an important change of pre-procedural decision making of the heart team, associated with favorable clinical outcomes. This prospective multicenter […]
EDWARDS ANNOUNCES ONE-YEAR DATA ON TRANSFEMORAL TRANSCATHETER TRICUSPID VALVE REPLACEMENT
LONDON, Nov. 27, 2022 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW) announced that one-year results on patients treated in the single-arm, prospective, global, multi-center TRISCEND study of the company’s EVOQUE transcatheter tricuspid valve replacement system demonstrated favorable safety, efficacy and quality-of-life outcomes. The data were presented during the late-breaking trials session at PCR London […]
Late-Breaking Post-Market Study Data Reinforce Clinical Procedural Success and Safety of the ACURATE neo2™ Aortic Valve System
MARLBOROUGH, Mass., Nov. 27, 2022 /PRNewswire/ — Boston Scientific Corporation (NYSE:BSX) has announced the first results from the ACURATE neo2 Post Market Clinical Follow-up (PMCF) study evaluating the performance of the ACURATE neo2TM Aortic Valve System. The findings, which included a high procedural success rate of 98.4% and low rates of mortality and paravalvular […]
Peijia Medical Limited and inQB8 Medical Technologies, LLC report Successful First-in-Human implantation of MonarQ Transcatheter Tricuspid Valve Replacement System
SUZHOU, China, Nov. 25, 2022 /PRNewswire/ — inQB8 Medical Technologies, LLC (inQB8), in Partnership with Peijia Medical Limited (Peijia, (9996.HK)), announced today that it has successfully implanted the MonarQ Transcatheter Tricuspid Valve in a 75-year-old female suffering from severe torrential Tricuspid Regurgitation. The Trans-Jugular TTVR procedure was performed on November 21, 2022, on compassionate grounds […]
Tenaya Therapeutics Receives Orphan Drug Designation from the U.S. Food and Drug Administration for its Gene Therapy for Genetic Arrhythmogenic Right Ventricular Cardiomyopathy
TN-401 is being developed for the treatment of genetic arrhythmogenic right ventricular cardiomyopathy (ARVC) caused by mutations in the PKP2 gene Orphan Drug Designation for TN-401 is the first for a gene therapy treatment for ARVC Expect to submit TN-401 IND application to the FDA in 2023 SOUTH SAN FRANCISCO, […]
CinCor Pharma Announces Topline Data for Phase 2 HALO Trial Evaluating Selective Aldosterone Synthase Inhibitor Baxdrostat in Uncontrolled Hypertension
Primary endpoint in Intention to Treat (ITT) was not met despite large absolute reductions in Systolic Blood Pressure (SBP) 12.6 mmHg placebo-adjusted reduction in SBP with 2 mg baxdrostat in a pre-specified subgroup that represents approximately 81-89% of the U.S. hypertension population (nominal p-value = 0.001) Safety profile and tolerability […]
Anteris Technologies Receives FDA Clearance to Initiate Early Feasibility Study for its Novel TAVR Product, DurAVR™
U.S. Clinical Trial Commencement Anticipated in 1Q2023 BRISBANE, Australia & EAGAN, Minn.–(BUSINESS WIRE)–Anteris Technologies Ltd (ASX: AVR), a structural heart company developing DurAVR™, the world’s only balloon-expandable, 3D single-piece aortic valve shaped to mimic the native human valve, today announced the U.S. Food and Drug Administration (FDA) has conditionally approved […]