CARLSBAD, Calif., Nov. 2, 2022 /PRNewswire/ — ImpediMed Limited (ASX: IPD) reached a milestone of 500,000 patient tests performed with the SOZO® Digital Health Platform. With over 1,100 devices in use globally, the pace of patient testing has accelerated with 100,000 patient tests performed in just the past six months. Product improvements, […]
Coronary/Structural Heart
Clario Extends Lead in Delivering Decentralized (DCT) and Hybrid Clinical Trials
New data shows Clario has helped more than 2.6 million patients participate in life changing clinical trials for innovative new medicines through decentralized and hybrid technology solutions More than 1000 compounds: Number of potential medicines tested using Clario remote data collection technologies Over 2.6 million patients: Number of participants in Clario DCTs […]
New Randomized Controlled Study Published in JAMA Demonstrates the Value of Clinical Decision Support Tools in Preventing Acute Kidney Injury in Patients Undergoing Coronary Angiography
– Researchers used Terumo Health Outcomes’ ePRISM® software to calculate safe contrast volume targets and improve overall patient outcomes – SOMERSET, N.J., Nov. 1, 2022 /PRNewswire/ — A new study published in JAMA[1] demonstrated that patients at high risk for acute kidney injury (AKI), the most common complication of percutaneous coronary intervention (PCI), had a significant […]
Baylis Medical Technologies Signs Distribution Agreement with Oscor
MISSISSAUGA, ON, Nov. 1, 2022 /PRNewswire/ – Baylis Medical Technologies Inc. is pleased to announce it is assuming responsibility for the sales and distribution of the Steerable and Guiding Sheaths currently sold direct-to-hospital by Oscor in the United States. The addition of Oscor’s sheath portfolio will allow Baylis Medical Technologies to broaden its […]
Temple Health Collaborates with Aidoc to Reduce Cost of Care using AI
Temple Health is introducing Aidoc’s suite of AI solutions which has demonstrated in clinical research to help appropriately reduce the patient length of stay, a metric strongly correlated with cost of patient care NEW YORK, Nov. 1, 2022 /PRNewswire/ — Aidoc, the leading provider of AI healthcare solutions, has announced a collaboration […]
Impella RP Flex with SmartAssist Receives FDA Approval to Treat Right Heart Failure
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (Nasdaq: ABMD) announces that Impella RP Flex with SmartAssist has received U.S. Food and Drug Administration (FDA) pre-market approval (PMA), the FDA’s highest level of approval, as safe and effective to treat acute right heart failure for up to 14 days. Impella RP Flex is implanted via the […]
Cleerly Introduces Proxy Solution to Improve Heart Disease Imaging Workflows
Proxy allows for seamless data transmission and improved AI-based plaque analysis NEW YORK–(BUSINESS WIRE)–Cleerly, the company creating a new standard of care for heart disease, announced a new workflow solution to its portfolio, Proxy. Cleerly Proxy is a software that allows Cleerly to seamlessly transmit data to care providers by […]
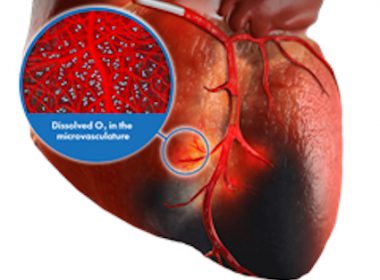
ZOLL Awarded Diagnostic and Interventional Cardiology Catheter Agreement with Premier, Inc. for TherOx SuperSaturated Oxygen Therapy
CHELMSFORD, Mass.–(BUSINESS WIRE)–ZOLL, an Asahi Kasei company that manufactures medical devices and related software solutions, announced today that its TherOx SuperSaturated Oxygen (SSO2) Therapy has been awarded a group purchasing agreement for Diagnostic and Interventional Cardiology Catheters (DIC) with Premier, Inc., effective as of July 1, 2022. The agreement allows […]
United Therapeutics Announces Top Line Data From the EXPEDITE Study of Remodulin Induction Prior to Orenitram Therapy
A short induction of Remodulin® (treprostinil) injection allowed study subjects to reach double the typical doses of Orenitram® (treprostinil) extended-release tablets than patients who did not have a Remodulin induction SILVER SPRING, Md. & RESEARCH TRIANGLE PARK, N.C.–(BUSINESS WIRE)–United Therapeutics Corporation (Nasdaq: UTHR), a public benefit corporation, today announced that a preliminary […]
Verve Therapeutics Announces Publication of VERVE-101 Preclinical Data in Circulation and Presentations at the American Heart Association Annual Meeting
Verve Researchers Awarded Paul Dudley White International Scholar Award from the American Heart Association for Highest Ranked Abstract Submitted from the United States to Scientific Sessions 2022 BOSTON, Oct. 31, 2022 (GLOBE NEWSWIRE) — Verve Therapeutics, Inc., a clinical-stage biotechnology company pioneering a new approach to the care of cardiovascular disease […]