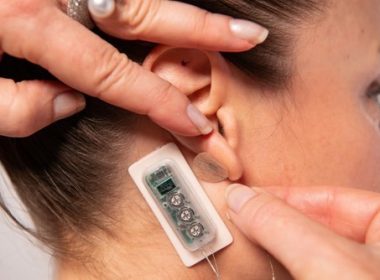
PALO ALTO, Calif., Sept. 22, 2022 /PRNewswire/ — DyAnsys, Inc., has announced that Primary Relief, a percutaneous electrical neurostimulation device, has been approved to treat pain following a cardiac surgery. The percutaneous electrical nerve stimulator (PENS) system can be used for up to three days for symptomatic relief of post-operative pain following […]
Coronary/Structural Heart
Vicore announces that C21 promotes vascular function in humans
Clinically relevant doses of C21 increase blood flow in humans Systemic blood pressure was unaffected, and no side effects were observed The forearm blood flow method is a robust technique for early clinical concept testing GOTHENBURG, Sweden, Sept. 22, 2022 /PRNewswire/ — Vicore Pharma Holding AB (publ) (“Vicore”), a clinical-stage pharmaceutical company developing […]
New Study Published in an American Heart Association Journal Indicates that Hello Heart’s Digital Coaching App Could Contribute to Heart Health Equity
The findings were published in the American Heart Association’s Hypertension journal and showed consistency of program results across diverse populations. MENLO PARK, Calif.–(BUSINESS WIRE)–Hello Heart, the only digital therapeutic that focuses exclusively on heart health, has released a new study evaluating the program’s results for various user population groups in order to demonstrate […]
Cardio Diagnostics Announces Issuance of U.S. Patent for Compositions and Methods for Detecting Predisposition to Cardiovascular Disease
CHICAGO, Sept. 21, 2022 /PRNewswire/ — Cardio Diagnostics, Inc. (“Cardio Diagnostics” or the “Company”), a pioneering precision cardiovascular testing company, today announced that the U.S. Patent and Trademark Office has issued to the University of Iowa Research Foundation (“UIRF”) U.S. Patent No. 11,414,704 titled COMPOSITIONS AND METHODS FOR DETECTING PREDISPOSITION TO CARDIOVASCULAR DISEASE, which is exclusively […]
Verve Therapeutics Announces Clearance of Clinical Trial Authorisation Application by the United Kingdom Medicines and Healthcare Products Regulatory Agency for VERVE-101 in Patients with Heterozygous Familial Hypercholesterolemia
CAMBRIDGE, Mass., Sept. 21, 2022 (GLOBE NEWSWIRE) — Verve Therapeutics, Inc., a clinical-stage biotechnology company pioneering a new approach to the care of cardiovascular disease with single-course gene editing medicines, today announced the clearance of its Clinical Trial Authorisation (CTA) application by the United Kingdom (U.K.) Medicines and Healthcare products Regulatory […]
Getinge partners with Medtronic for the manufacturing of the Radiant[TM] balloon-expandable covered stent
Getinge announces a supply partnership with Medtronic, which recently received CE mark for the Radiant™ covered stent, the first covered stent indicated for chimney endovascular aneurysm repair (ChEVAR). The Radiant covered stent is a private labeled Advanta V12 balloon expandable covered stent produced by Getinge and distributed by Medtronic. The […]
Pulnovo Medical Released Results From PADN-CFDA Pivotal Trial For The Treatment Of Pulmonary Arterial Hypertension (PAH)
SHANGHAI, Sept. 20, 2022 /PRNewswire/ — Pulnovo Medical Limited, a globally recognized OTM platform, today released the positive results from the Pulmonary Artery Denervation (PADN)-CFDA pivotal study on Transcatheter Cardiovascular Therapy conference (TCT 2022),one of the world’s most influential cardiovascular conference. PADN is an innovative radiofrequency ablation technique in treating PH, was recognized as […]
Neovasc Provides Corporate Update Following Clinical Data Release
New Data Presented on the Neovasc Reducer™ in Patients With Microvascular Disease VANCOUVER and MINNEAPOLIS, Sept. 20, 2022 (GLOBE NEWSWIRE) — via NewMediaWire – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ, TSX: NVCN) announced positive clinical data at the Transcatheter Cardiovascular Therapeutics Conference (“TCT”) held September 16-19 in Boston. Prof. Shmuel Banai, MD, Neovasc […]
Orchestra BioMed™ Announces Presentation of Positive Long-Term Clinical Results from MODERATO II Control Patients Crossover Extension Study of BackBeat CNT™ at TCT 2022
Statistically significant mean reductions of 10.3 mmHg in 24-hour ambulatory systolic blood pressure and 11.7 mmHg in ambulatory pulse pressure after 6 months of therapy in control patients who crossed over to BackBeat CNT following completion of the randomized portion of the MODERATO II study NEW HOPE, Pa., Sept. 20, […]
Otsuka Medical Devices Announces Results of RADIANCE II Pivotal Trial at TCT 2022 Annual Meeting
Study of the Paradise™ Ultrasound Renal Denervation System Shows significant Reduction in Blood Pressure in Patients with Uncontrolled Hypertension TOKYO–(BUSINESS WIRE)–Otsuka Medical Devices Co., Ltd. (“Otsuka Medical Devices”) a wholly owned subsidiary of Otsuka Holdings today announced the detailed results from the RADIANCE II US FDA IDE pivotal trial evaluating […]