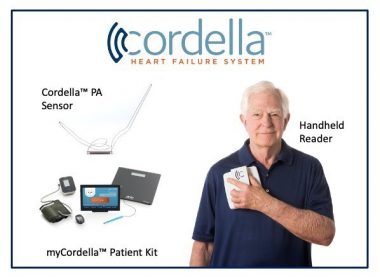
Study met all primary safety and efficacy endpoints for the Cordella™ PA Pressure Sensor System for the treatment of NYHA class III heart failure patients LISLE, Ill., May 23, 2022 /PRNewswire/ — Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure (HF), today announced positive data […]
Coronary/Structural Heart
Axon Therapies Announces Positive Early Clinical Data from REBALANCE-HF Randomized Clinical Trial
Late-breaking results presented at the European Society of Cardiology’s Heart Failure Association annual conference and simultaneously published online in the European Journal of Heart Failure SANTA CLARA, Calif., May 23, 2022 /PRNewswire/ — Axon Therapies, a private company focused on addressing a root cause of heart failure, today announced positive early results from […]
Evkeeza® (evinacumab) Phase 3 Trial Demonstrates 48% LDL-C Reduction in Children with Ultra-rare Form of High Cholesterol
Children already on other lipid-lowering therapies entered the trial with dangerously high LDL-C (264 mg/dL on average), and 79% saw their LDL-C reduced by at least half at 24 weeks FDA submission planned by end of 2022 Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN) today announced positive results from a Phase 3 trial evaluating Evkeeza® (evinacumab) […]
Shockwave Medical and Genesis MedTech Obtain Regulatory Approval in China for Intravascular Lithotripsy
Approvals Cover Both Coronary and Peripheral IVL Catheters and Generators Manufactured by Shockwave SANTA CLARA, Calif. and SINGAPORE, May 23, 2022 (GLOBE NEWSWIRE) — Shockwave Medical, Inc. (NASDAQ: SWAV), a pioneer in the development of Intravascular Lithotripsy (IVL) to treat severely calcified cardiovascular disease, and Genesis MedTech Group announced today that […]
Cytokinetics Announces Data From REDWOOD-HCM OLE and GALACTIC-HF Presented as Late Breaking Science Presentations at the European Society of Cardiology Heart Failure 2022 Congress
Data from Up to Six Months of Treatment with Aficamten Show Significant and Sustained Reductions in LVOT Gradients with Improvements in Functional Class, Symptoms, and Biomarkers Analyses from GALACTIC-HF Show Patients with Low Blood Pressure Have Increased Treatment Effect SOUTH SAN FRANCISCO, Calif., May 23, 2022 (GLOBE NEWSWIRE) — Cytokinetics, […]
Recardio’s Positive Phase 2 Results Presented as Latebreaker at World Congress on Acute Heart Failure 2022 in Madrid
Recardio Inc., a clinical-stage life science company developing regenerative therapies for cardiovascular diseases, announced that the positive study results of Recardio’s Phase 2 clinical study in Acute Myocardial Infarction were presented on Saturday, May 21, 2022, during the Latebreaker Session at the World Congress on Acute Heart Failure 2022 in […]
Windtree Presents Data from its Positive SEISMiC Phase 2 Study of Istaroxime in Early Cardiogenic Shock in a Late-Breaker Presentation at the European Society of Cardiology Heart Failure Meeting in Madrid
The study met its primary endpoint of improved systolic blood pressure (SBP) profile at 6 hours with the istaroxime group performing significantly better than the control group Increased SBP also persisted through 24 hours The study also met several key secondary endpoints associated with improving cardiac function Investor conference call […]
Positive Phase 3 study with aprocitentan demonstrates significant antihypertensive efficacy in patients with resistant hypertension
Ad hoc announcement pursuant to Art. 53 LR Aprocitentan reduces blood pressure compared to placebo by week 4 of treatment, the effect is maintained and confirmed over a period of 48 weeks, and is generally well tolerated with no major safety concerns Primary and key secondary endpoints were met with […]
Late-breaking Results Show Efficacy for CCM in Patients With HFpEF
Investigators Report Positive Findings From Feasibility Study of CCM in Heart Failure With Preserved Ejection Fraction at the European Society of Cardiology Heart Failure 2022 Congress MADRID and MARLTON, N.J., May 23, 2022 (GLOBE NEWSWIRE) — Impulse Dynamics, a global medical device company dedicated to improving the lives of people […]
Alnylam Presents New 18-Month Results from Exploratory Cardiac Endpoints in HELIOS-A Phase 3 Study of Investigational Vutrisiran
– New Exploratory Findings from 18-Month Analysis of HELIOS-A Suggest that Treatment with Investigational Vutrisiran May Benefit Patients with Hereditary ATTR (hATTR) Amyloidosis and Cardiac Involvement – Improvements in NT-proBNP and Echocardiographic Parameters Seen in Cardiac Subpopulation – Reductions in Technetium Uptake Observed in Patients with High Grade Uptake at […]