– Results were published in JACC: Basic to Translational Science – – Publication evaluated coronary tissue engineered vessel (CTEV) as a conduit for CABG in a nonhuman primate model – – All implanted CTEVs remained patent through six months, demonstrated adaptive remodeling, and recellularized with host coronary artery cells – – Humacyte plans to advance CTEV into first-in-human study in CABG – DURHAM, N.C., Sept. 18, 2025 (GLOBE NEWSWIRE) — Humacyte, Inc. (Nasdaq: HUMA), a commercial-stage biotechnology platform company developing universally implantable, bioengineered human tissues at commercial scale, today announced the publication of new preclinical data as part of a study evaluating the coronary tissue engineered vessel (CTEV) as a coronary artery bypass graft conduit in a non-human primate model. In the study, published in JACC: Basic to Translational Science, a specialist journal launched by the Journal of the American College of Cardiology (JACC), the CTEV was observed to sustain blood flow, recellularize with the animals’ host cells, and remodel to reduce the initial size mismatch between the CTEV and the animals’ native artery. “Innovation in CABG has been stagnant for far too long,” said Alan Kypson, MD, FACS, Cardiothoracic Surgeon, UNC REX Hospital. “Our results suggest that we may be on the verge of a new option — one that remodels to match the native artery and recellularizes with host cells, potentially providing superior patency relative to saphenous vein grafts. The CTEV has the potential to address a significant unmet clinical need in coronary bypass surgery and ultimately improve patient outcomes.” Cardiovascular disease is the leading cause of death worldwide, comprising one in every three deaths in the United States in 2023. The most common form is coronary artery disease (CAD), which affects 1 in 20 adults aged 20 and older. Coronary artery bypass grafting, a procedure that improves blood flow to the heart by using the internal mammary artery and saphenous vein to bypass narrowed or blocked coronary arteries, remains the current standard of care. However, saphenous vein grafts – used in 80%-90% of CABG cases – demonstrate poor long-term patency, with approximately 50% failing at 10 years. Many patients also lack usable autologous veins or arteries due to prior harvest, ablation, or poor quality, highlighting the unmet clinical need for alternative conduits. For the past half century, no novel CABG conduits have gained routine clinical use. The recent JACC study, titled “Acellular Tissue Engineered Vessels as Coronary Artery Bypass Grafts”, follows five adult baboons undergoing CABG to the right coronary artery with the CTEV. All CTEVs remained patent throughout the 6-month study. At the end of follow-up, the CTEV was observed to have recellularized with host cells to form a multi-layered tissue, including transanastomotic neomedial tissue that effectively reduced the initial size mismatch with the right coronary artery (RCA). The results suggest that the CTEV may be a durable alternative CABG conduit. The CTEV is 3.5mm blood vessel produced in the same bioengineering manufacturing system as Humacyte’s acellular tissue engineered vessel (ATEV™). The CTEV is also referred to as the small-diameter ATEV, or sdATEV. Humacyte also announced that it plans to advance the CTEV into its first-in-human study in CABG. To support human study, the Company anticipates filing an Investigational New Drug (IND) application with the U.S. Food and Drug Administration (FDA) during the 4th Quarter of 2025. The Company’s current plans for filing an IND are based on the outcome of a meeting held early this year with the FDA, including agreements reached with the agency. “We’re pleased that this new publication of preclinical data demonstrates the promise of CTEVs as an alternative for native vessel grafts in CABG,” said Laura Niklason, M.D., Ph.D., Founder and Chief Executive Officer of Humacyte. “As one of the leading causes of early death, coronary artery disease poses unique challenges for patient care. We are looking forward to proceeding into the first-in-human study of the CTEV in CABG and hopefully offering surgeons another option for treating this disease.” The CTEV is an investigational product and has not been approved for sale by the FDA or any other regulatory agency. About Humacyte Humacyte, Inc. (Nasdaq: HUMA) is developing a disruptive biotechnology platform to deliver universally implantable bioengineered human tissues, advanced tissue constructs, and organ systems designed to improve the lives of patients and transform the practice of medicine. The Company develops and manufactures acellular tissues to treat a wide range of diseases, injuries, and chronic conditions. Humacyte’s Biologics License Application for the acellular tissue engineered vessel (ATEV) in extremity vascular trauma was approved by the FDA in December 2024. ATEVs are also currently in late-stage clinical trials targeting other vascular applications, including arteriovenous (AV) access for hemodialysis and peripheral artery disease (PAD). Preclinical development is also underway in coronary artery bypass grafts, pediatric heart surgery, treatment of type 1 diabetes, and multiple novel cell and tissue applications. Humacyte’s 6mm ATEV for AV access in hemodialysis was the first product candidate to receive the FDA’s Regenerative Medicine Advanced Therapy (RMAT) designation and has also received FDA Fast Track designation. Humacyte’s 6mm ATEV for urgent arterial repair following extremity vascular trauma and for advanced PAD also have received RMAT designations. The ATEV received priority designation for the treatment of vascular trauma by the U.S. Secretary of Defense. For more information, visit www.Humacyte.com. For uses other than the FDA approval in the extremity vascular trauma indication, the ATEV is an investigational product and has not been approved for sale by the FDA or any other regulatory agency. Forward-Looking Statements This press release contains forward-looking statements that are based on beliefs and assumptions and on information currently available. In some cases, you can identify forward-looking statements by the following words: “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties, and other factors that may cause actual results, levels of activity, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this press release, we caution you that these statements are based on a combination of facts and factors currently known by us and our projections of the future, about which we cannot be certain. Forward-looking statements in this press release include, but are not limited to, our plans and ability to commercialize Symvess and, if approved by regulatory authorities, our product candidates, successfully and on our anticipated timelines; the degree of market acceptance of and the availability of third-party coverage and reimbursement for Symvess and, if approved by regulatory authorities, our product candidates; our ability to manufacture Symvess and, if approved by regulatory authorities, our product candidates in sufficient quantities to satisfy our clinical trial and commercial needs; the anticipated benefits of our ATEVs and our CTEVs relative to existing alternatives; our plans and ability to execute product development, process development and preclinical development efforts successfully and on our anticipated timelines; our ability to design, initiate and successfully complete clinical trials and other studies for our product candidates and our plans and expectations regarding our ongoing or planned clinical trials; the anticipated characteristics and performance of our ATEVs and our CTEVs; the implementation of our business model and strategic plans for our business; our ability to execute and achieve the expected benefits of our cost-saving measures and whether our efforts will result in further actions or additional asset impairment charges that adversely affect our business; and the timing or likelihood of regulatory filings, acceptances and approvals. We cannot assure you that the forward-looking statements in this press release will prove to be accurate. These forward-looking statements are subject to a number of significant risks and uncertainties that could cause actual results to differ materially from expected results, including, among others, changes in applicable laws or regulations, the possibility that Humacyte may be adversely affected by other economic, business, competitive and/or reputational factors, and other risks and uncertainties, including those described under the header “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2024 and Form 10-Q for the quarter ended June 30, 2025, each filed by Humacyte with the SEC, and in future SEC filings. Most of these factors are outside of Humacyte’s control and are difficult to predict. Furthermore, if the forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. Except as required by law, we have no current intention of updating any of the forward-looking statements in this press release. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this press release. Humacyte Investor Contact:Joyce AllaireLifeSci Advisors LLC+1-617-435-6602jallaire@lifesciadvisors.cominvestors@humacyte.com Humacyte Media Contact:Rich LuchettePrecision Strategies+1-202-845-3924rich@precisionstrategies.commedia@humacyte.com
Coronary/Structural Heart
Novo Nordisk A/S: Ozempic® reduces the risk of heart attack, stroke and death by 23% compared to dulaglutide in the first head-to-head real-world study
Ozempic® (once-weekly injectable semaglutide) was associated with a 23% reduced risk of heart attack, stroke and death in people with type 2 diabetes and cardiovascular disease on Medicare versus dulaglutide1Data also highlighted Ozempic® was associated with a 26% lower risk of death versus dulaglutide1This study is the first to directly compare these glucagon-like peptide 1 receptor agonist (GLP-1 RA) medications in everyday real-life use. It fills a significant gap in understanding their effects on heart health in older people at high risk, which could help support informed treatment decisions and health policies.1 Bagsværd, Denmark, 18 September 2025 – Novo Nordisk today announced results from the REACH real-world study, which demonstrated that compared to dulaglutide, Ozempic® (once-weekly injectable semaglutide) was associated with a reduced risk of major adverse cardiovascular events such as a heart attack or stroke by 23%1. The data span nearly 60,000 US Medicare patients (aged ≥66 years) living with type 2 diabetes, atherosclerotic cardiovascular disease (ASCVD) – a condition where fatty deposits build up in blood vessels, reducing blood flow and increasing the risk of heart attacks, strokes and related problems – and multiple health conditions2. The results were presented at the European Association for the Study of Diabetes (EASD) 2025 Annual Meeting on 15–19 September in Vienna, Austria1. “As we age, the risk of experiencing a heart attack, stroke or dying from a cardiovascular event increases. At the same time, there are limited clinical data for people living with diabetes and cardiovascular disease aged 66 years or older. These data, showing a 23% risk reduction of a heart attack, stroke and death, fill an important gap and reinforce the well-established clinical evidence of semaglutide,” said Filip Krag Knop, senior vice president and incoming chief medical officer at Novo Nordisk. “This is great news for older patients as well as healthcare professionals, as these results build on the importance of our randomised clinical trial data assessing the effectiveness of treatments in a real-life setting. This also supports what we already know from our clinical development programmes that not all GLP-1 RAs are the same.”Beyond these essential benefits, once-weekly semaglutide was also associated with a 25% risk reduction of heart attack, stroke, hospitalisation for unstable angina or heart failure, and death from any cause (five-point MACE)1. Ozempic® is the only GLP-1 RA that has proven risk reduction of cardiovascular and kidney events in people with type 2 diabetes3-6. These results provide the first direct comparison of cardiovascular outcomes between Ozempic® and dulaglutide in US Medicare beneficiaries and add to the body of evidence for Ozempic®. About REACH REACH is a comprehensive series of studies assessing the cardiovascular disease-related outcomes of the once-weekly GLP-1 RA class, including semaglutide, compared to glucose-lowering therapies such as DPP4 inhibitors, SGLT2 inhibitors and others. It also includes within-class comparisons of GLP-1 RAs from multiple administrative claims and electronic health record databases. The study presented at the European Association for the Study of Diabetes (EASD) 2025 Annual Meeting is a real-world evidence analysis evaluating cardiovascular risk reduction with GLP-1 RAs – semaglutide and dulaglutide – in people with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD). This study draws from Medicare fee-for-service claims data, using a target-trial emulation framework, including 58,336 matched patients (29,168 patients in each treatment group) aged ≥66 years with type 2 diabetes and ASCVD who initiated once-weekly semaglutide or dulaglutide. Direct cardiovascular outcome comparisons between GLP-1 RAs are lacking. These results address a critical gap for insights, particularly in an older Medicare population with multiple comorbidities underrepresented in randomised clinical trials. About Ozempic®Ozempic® (semaglutide) injection 0.25 mg, 0.5 mg, 1.0 mg or 2.0 mg is a once-weekly GLP-1 RA indicated, along with diet and exercise, to improve blood sugar (glucose) in adults with type 2 diabetes and to reduce the risk of major cardiovascular events such as heart attack, stroke or death in adults with type 2 diabetes mellitus with known heart disease. Ozempic® is the only GLP-1 RA indicated to reduce the risk of worsening kidney disease and risk of death from cardiovascular events in adults with type 2 diabetes and chronic kidney disease. Ozempic® is currently marketed in 72 countries, and 7 million people with type 2 diabetes are currently being treated with Ozempic® worldwide. Novo Nordisk is a leading global healthcare company founded in 1923 and headquartered in Denmark. Our purpose is to drive change to defeat serious chronic diseases built upon our heritage in diabetes. We do so by pioneering scientific breakthroughs, expanding access to our medicines, and working to prevent and ultimately cure disease. Novo Nordisk employs about 78,400 people in 80 countries and markets its products in around 170 countries. For more information, visit novonordisk.com, Facebook, Instagram, X, LinkedIn and YouTube. Contacts for further information Media: Ambre James-Brown +45 3079 9289 globalmedia@novonordisk.com Liz Skrbkova (US) +1 609 917 0632 lzsk@novonordisk.com Investors: Jacob Martin Wiborg Rode +45 3075 5956 jrde@novonordisk.com Sina Meyer +45 3079 6656 azey@novonordisk.com Max Ung +45 3077 6414 mxun@novonordisk.com Christoffer Sho Togo Tullin +45 3079 1471 cftu@novonordisk.com Alex Bruce +45 34 44 26 13 axeu@novonordisk.com Frederik Taylor Pitter +1 609 613 0568 fptr@novonordisk.com _______________________References1. Tan M, et al. Late-breaking oral presentation presented at the European Association for the Study of Diabetes (EASD) 2025; Sep 15-19 2025; Vienna, Austria.2. Dai D, et al. Cancer Control. 2022;29:10732748221140691.3. Marso SP, et al. N Engl J Med. 2016;375:1834-1844.4. Perkovic V, et al. N Engl J Med. 2024;391:109-121.5. Ozempic® (once-weekly semaglutide): US Prescribing Information. 2025 [online]. Available at: https://www.novo-pi.com/ozempic.pdf Last accessed: September 2025.6. EMA. Ozempic® (once-weekly semaglutide) Summary of Product Characteristics. 2025 [online]. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/ozempic. Last accessed: September 2025.
Attachment
PR250918-EASD-REACH
NobleStitch™ Suture-Mediated PFO Closure Demonstrates Superior Long-Term Safety and Outcomes in Largest Cohort to Date
New data show zero device-related complications or atrial fibrillation, no recurrent stroke or TIA, with a cohort 40% larger than Gore and Amplatzer device studies FOUNTAIN VALLEY, Calif., Sept. 16, 2025 /PRNewswire/ — NobleStitch™, the deviceless suture-mediated patent foramen ovale…
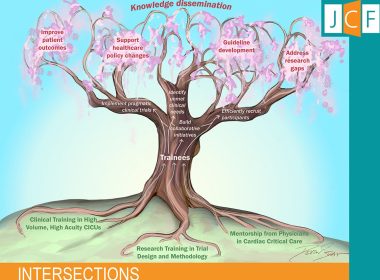
First Issue of New HFSA Journal of Cardiac Failure-Intersections
The first issue of the Journal of Cardiac Failure-Intersections, an open-access journal focused on heart failure and its many intersections, is now available. WASHINGTON, Sept. 17, 2025 /PRNewswire/ — Scientific breakthroughs often begin with a single Aha! Moment—an idea that evolves…
Tenax Therapeutics Announces European Patent Office Intention to Grant Patent Covering Use of Levosimendan in PH-HFpEF
Provides Patent Protection in Europe Through at Least December 2040 Further IP Protection for TNX-103 (Oral Levosimendan) and Other Drug Formulations, and its Active Metabolites, in PH-HFpEF Also Provides Patent Protection for Wide Range of Levosimendan Doses, and its Use in Combination with Various Cardiovascular Drugs in PH-HFpEF CHAPEL HILL, N.C., Sept. 16, 2025 (GLOBE NEWSWIRE) — Tenax Therapeutics, Inc. (Nasdaq: TENX) (“Tenax” or “Tenax Therapeutics” or the “Company”), a Phase 3, development-stage pharmaceutical company using clinical insights to develop novel cardiopulmonary therapies, today announced that the European Patent Office (EPO) has notified Tenax of its Intention to Grant a patent that will provide intellectual property (IP) protection for TNX-103 (oral levosimendan), and other formulations of levosimendan, as well as its active metabolites, for use in pulmonary hypertension resulting from heart failure with preserved ejection fraction (PH-HFpEF). Once granted, this patent will provide Tenax with protection in Europe through 2040, and may qualify for an additional European patent term beyond 2040. “This patent will protect our use of levosimendan in PH-HFpEF, including TNX-103, in Europe, where prevalence estimates indicate the number of patients currently suffering from this disease approximates the number estimated in North America. This patent protects an important commercial opportunity for Tenax. It builds on multiple patents already issued in the U.S. and Canada, as we continue to strengthen the global IP portfolio of our novel and potentially first-in-disease drug,” said Chris Giordano, President and Chief Executive Officer of Tenax Therapeutics. “The European patent is particularly timely for the Company as we prepare to initiate our second registrational Phase 3 study, LEVEL-2, which will recruit patients across Europe. We expect to initiate LEVEL-2 this year, an essential step not only in advancing TNX-103 toward regulatory approval in PH-HFpEF, but also to including patients from around the world at the Phase 3 stage of development.” The EPO issued an Intention to Grant notification for Tenax’s patent application titled “LEVOSIMENDAN FOR TREATING PULMONARY HYPERTENSION WITH HEART FAILURE WITH PRESERVED EJECTION FRACTION (PH-HFpEF).” Once granted, this patent will include claims covering the use of levosimendan when delivered through various routes of administration to treat PH-HFpEF, including oral, intravenous, inhaled, transdermal, and subcutaneous use. In addition, this patent will include similar protection for the use of the active metabolites of levosimendan (OR1896 and OR1855) for the treatment of PH-HFpEF. This patent also will expressly provide protection in Europe for a wide range of doses of levosimendan, as well as for use of levosimendan in combination with various cardiovascular drugs, in PH-HFpEF. This new patent, once granted, will have a patent term until December 2040, and it may qualify for European patent supplementary protection certificates (SPC) that would extend the period of patent protection beyond 2040. About Levosimendan (TNX-101, TNX-102, TNX-103) Levosimendan is a novel, first-in-class K-ATP channel activator/calcium sensitizer currently being evaluated to treat pulmonary hypertension (PH) associated with heart failure with preserved ejection fraction (PH-HFpEF). Levosimendan was first developed for intravenous use in hospitalized patients with acutely decompensated heart failure, and it has received market authorization in 60 countries in this indication, although it is not available in the United States or Canada. Tenax’s Phase 2 HELP study, including its open-label extension stage, demonstrated the potential of IV (TNX-101) and oral (TNX-103) levosimendan to bring durable improvements in exercise capacity and quality of life, as well as other clinical assessments, in patients with PH-HFpEF. TNX-103 (oral levosimendan) is currently being evaluated in LEVEL, a Phase 3, double-blind, randomized, placebo-controlled clinical trial in patients with PH-HFpEF. About Tenax Therapeutics Tenax Therapeutics, Inc. is a Phase 3, development-stage pharmaceutical company using clinical insights to develop novel cardiopulmonary therapies. The Company owns global rights to develop and commercialize levosimendan, which it is developing for the treatment of PH-HFpEF, the most prevalent form of pulmonary hypertension globally, for which no product has been approved to date. For more information, visit www.tenaxthera.com. Tenax Therapeutics’ common stock is listed on The Nasdaq Stock Market LLC under the symbol “TENX”. Caution Regarding Forward-Looking Statements Except for historical information, all of the statements, expectations and assumptions contained in this press release are forward-looking statements. These forward-looking statements may include information concerning expected intellectual property developments and possible or projected future business operations. Actual results might differ materially from those explicit or implicit in the forward-looking statements. Important factors that could cause actual results to differ materially include: intellectual property risks; risks of our clinical trials, including, but not limited to, the timing, delays, costs, design, location, initiation, enrollment, and results of such trials; any delays in regulatory review and approval of product candidates in development; risks related to our business strategy, including the prioritization and development of product candidates; our estimates regarding the potential market opportunity for our product candidates; reliance on third parties, including Orion Corporation, our manufacturers and CROs; risks regarding the formulation, production, marketing, customer acceptance and clinical utility of our product candidates; the potential advantages of our product candidates; our competitive position; our ability to maintain our culture and recruit, integrate and retain qualified personnel and advisors, including on our Board of Directors; risks associated with our cash needs; volatility and uncertainty in the global economy and financial markets in light of unexpected changes in tariffs and the possibility of pandemics, global financial and geopolitical uncertainties, including in the Middle East and the Russian invasion of and war against the country of Ukraine; changes in legal, regulatory and legislative environments in the markets in which we operate and the impact of these changes on our ability to obtain regulatory approval for our products; and other risks and uncertainties set forth from time to time in our SEC filings. Tenax Therapeutics assumes no obligation and does not intend to update these forward-looking statements except as required by law. Contact: Investor and Media: Argot Partnerstenax@argotpartners.com
Caristo Congratulates Abcentra for First Patient Dosed Successfully in Phase 2b ‘FORTIFY’ Clinical Trial Evaluating Orticumab in Patients with Cardiovascular Disease
‘FORTIFY’ Selects FAI-Score™ as Primary Outcome Measure, and Caristo as Worldwide Imaging Core Lab for Coronary Inflammation and Plaque Measurement and Monitoring STAMFORD, Conn. and LOS ANGELES, Sept. 16, 2025 /PRNewswire/ — Caristo Diagnostics, on a mission to transform the diagnosis…
Conavi Medical Submits Next Generation Novasight Hybrid Intravascular Imaging System to US FDA for 510(k) Clearance
TORONTO, Sept. 16, 2025 (GLOBE NEWSWIRE) — Conavi Medical Corp. (TSXV: CNVI) (OTCQB: CNVIF) (“Conavi” or the “Company”), a commercial-stage medical device company focused on designing, manufacturing, and marketing imaging technologies to guide minimally invasive cardiovascular procedures, announced that it has submitted its next generation Novasight Hybrid™ IVUS/OCT intravascular imaging system to the U.S. Food and Drug Administration (“FDA”) for 510(k) clearance for coronary applications.
EU approval makes Novo Nordisk’s oral semaglutide the first and only oral GLP-1 RA to reduce cardiovascular death, heart attack and stroke
Oral semaglutide (Rybelsus®) is now the first and only oral GLP-1 RA approved for type 2 diabetes, with proven cardiovascular benefits1This approval is based on results from the SOUL clinical trial, where oral semaglutide (Rybelsus®) reduced cardiovascular death, heart attack and stroke by 14% versus placebo, when added to standard of care, in adults with type 2 diabetes at high cardiovascular risk1In addition, new results from SOUL will be presented at one of the largest diabetes conferences (EASD) later this week, showing that oral semaglutide significantly reduced hospitalisations compared with placebo2 Bagsværd, Denmark, 15 September 2025 – Novo Nordisk today announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has approved an update to the Rybelsus® (oral semaglutide) label to reflect the cardiovascular benefits seen in the SOUL trial. SOUL was a phase 3b trial carried out to evaluate the effect of Rybelsus® on cardiovascular outcomes in people with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) and/or chronic kidney disease (CKD)1. Rybelsus® is now the first and only oral glucagon-like peptide 1 receptor agonist (GLP-1 RA) – mimicking a natural hormone in your body that helps regulate blood sugar, appetite, and digestion – available in the EU for type 2 diabetes with a proven cardiovascular benefit1. “Heart problems are the leading cause of disability and death for people living with type 2 diabetes. Therefore, treatments that also address heart problems are key to improving not only health outcomes, but also quality of life – and this approval will help do just that,” said Emil Kongshøj Larsen, executive vice president, International Operations at Novo Nordisk. “This milestone makes semaglutide the only oral GLP-1 RA with proven blood glucose and body weight reduction, as well as cardiovascular benefits.” New results from the SOUL trial will be shared later this week at the European Association for the Study of Diabetes (EASD) 2025 Annual Meeting, 15–19 September. These include findings that treatment with oral semaglutide significantly reduced hospitalisations related to serious adverse events compared with placebo2. Additional SOUL results will be presented at the same meeting, which highlight that the cardiovascular benefits of oral semaglutide were consistent regardless of body mass index (BMI) and body weight of participants3. In the US, a decision is expected later this year for a label extension for the cardiovascular indication for Rybelsus®. Novo Nordisk has also submitted an application in the US for a once-daily 25 mg oral formulation of semaglutide (Wegovy® in a pill) in adults living with obesity or overweight and cardiovascular disease. A decision is expected at the turn of this year, and if approved, Wegovy® would become the first oral GLP-1 RA indicated for chronic weight management. Rybelsus® is the first and only oral GLP-1 RA approved for the treatment of type 2 diabetes, following its launch in 2019. It is supported by a strong clinical and real-world evidence base, demonstrating superior blood glucose reduction and body weight reduction versus multiple comparators, as well as an established safety profile in people with type 2 diabetes4-8. About SOUL SOUL was a multicentre, international, randomised, double-blind, parallel-group, placebo-controlled, phase 3 cardiovascular outcomes trial, with 9,650 participants enrolled. It was conducted to assess the effect of oral semaglutide versus placebo, when added to standard of care, on cardiovascular outcomes in people with type 2 diabetes and established cardiovascular disease and/or chronic kidney disease (CKD). The SOUL trial was initiated in 2019. The primary outcome was time-to-first occurrence of major adverse cardiovascular events (MACE; a composite objective consisting of cardiovascular death, heart attack and stroke) 1. The SOUL trial demonstrated a superior reduction in MACE of 14% for people treated with oral semaglutide compared to placebo in people with type 2 diabetes and cardiovascular disease and/or CKD, making Rybelsus® (oral semaglutide) the first and only oral GLP-1 RA with a proven cardiovascular benefit1. About Rybelsus® Rybelsus® (oral semaglutide) is a glucagon-like peptide 1 receptor agonist (GLP-1 RA) indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus to improve glycaemic control as an adjunct to diet and exercise7, 9. Rybelsus® is administered once daily and is approved for use in the following therapeutic dosages: 1.5 mg, 3 mg, 4 mg, 7 mg, 9 mg, 14 mg, 25 mg and 50 mg4, 5. Rybelsus® offers superior blood glucose lowering versus multiple comparators4, 5, together with consistent weight reduction4, 5, 10, reduction in cardiometabolic risk factors10 and reduction in major adverse cardiovascular events (MACE) 1. Rybelsus® is now the first and only oral GLP-1 RA available in the EU for type 2 diabetes with a proven cardiovascular benefit1. It is currently available in 48 countries11, and more than 2.4 million people with type 2 diabetes are currently being treated with Rybelsus® worldwide12. About Wegovy®Semaglutide 2.4 mg is marketed under the brand name Wegovy®. In the EU, Wegovy® is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management in adults with a BMI of 30 kg/m2 or greater (obesity) or adults with a BMI of 27 kg/m2 or greater (overweight) in the presence of at least one weight-related comorbid condition. In the EU, Wegovy® is also indicated for paediatric patients aged 12 years and older with an initial BMI at the 95th percentile or greater for age and gender (obesity) and body weight above 60 kg. The clinical section of the label also includes data on Wegovy® major adverse cardiovascular events (MACE) risk reduction, improvements in heart failure with preserved ejection fraction (HFpEF)-related symptoms and physical function, as well as pain reduction related to knee osteoarthritis13. In the US, Wegovy® is indicated in combination with a reduced calorie diet and increased physical activity to reduce the risk of MACE in adults with established cardiovascular disease and either obesity or overweight, to reduce excess body weight and maintain weight reduction long term in paediatric patients aged 12 years and older with obesity and in adults with obesity or with overweight in the presence of at least one weight-related comorbid condition, as well as for the treatment of noncirrhotic metabolic dysfunction-associated steatohepatitis (MASH) in adults with moderate to advanced liver scarring (fibrosis), but not with cirrhosis of the liver, in combination with a reduced calorie diet and increased physical activity14. Novo Nordisk is a leading global healthcare company founded in 1923 and headquartered in Denmark. Our purpose is to drive change to defeat serious chronic diseases built upon our heritage in diabetes. We do so by pioneering scientific breakthroughs, expanding access to our medicines, and working to prevent and ultimately cure disease. Novo Nordisk employs about 78,400 people in 80 countries and markets its products in around 170 countries. For more information, visit novonordisk.com, Facebook, Instagram, X, LinkedIn and YouTube. Contacts for further information Media: Ambre James-Brown +45 3079 9289globalmedia@novonordisk.comLiz Skrbkova (US)+1 609 917 0632lzsk@novonordisk.comInvestors: Jacob Martin Wiborg Rode+45 3075 5956jrde@novonordisk.comSina Meyer +45 3079 6656azey@novonordisk.comMax Ung+45 3077 6414 mxun@novonordisk.com Christoffer Sho Togo Tullin+45 3079 1471cftu@novonordisk.comAlex Bruce +45 34 44 26 13axeu@novonordisk.comFrederik Taylor Pitter +1 609 613 0568fptr@novonordisk.com _______________________References1. McGuire DK, et al. N Engl J Med. 2025;392(20):2001-2012.2. Buse JB, et al. Oral presentation presented at the European Association for the Study of Diabetes (EASD) 2025; 15-19 Sep 2025; Vienna, Austria.3. Inzucchi SE, et al. Oral presentation presented at the European Association for the Study of Diabetes (EASD) 2025; 15–19 Sep 2025; Vienna, Austria. 4. Rosenstock J, et al. JAMA. 2019;321(15):1466-1480.5. Rodbard HW, et al. Diabetes Care. 2019;42(12):2272-2281.6. Pratley R, et al. Lancet. 2019;394(10192):39-50.7. Rybelsus® (oral semaglutide): US Prescribing Information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/213051s023lbl.pdf. Last accessed: September 2025.8. Aroda VR, et al. Lancet. 2023;402(10403):693-704.9. Rybelsus® (oral semaglutide): Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/rybelsus. Last accessed: September 2025.10. Husain M, et al. N Engl J Med. 2019;381(9):841-851.11. Novo Nordisk Data on File. LEA portal Product Planning, 25th Aug 2025.12. Novo Nordisk Data on File. IQVIA Jun’25 Patients R3M Vol. data. 2025.13. Wegovy® (semaglutide): Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/wegovy-epar-product-information_en.pdf. Last accessed: September 2025.14. Wegovy® (semaglutide): US Prescribing Information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/215256s015lbl.pdf. Last accessed: September 2025.
Attachment
PR250915-Rybelsus-label-update-SOUL
Cadrenal Therapeutics Enhances Anticoagulation Pipeline Through Acquisition of eXIthera’s Portfolio of Factor XIa Inhibitors
Acquisition significantly enhances the Company’s pipeline by adding novel assets in acute and chronic anticoagulation settingsCompany is strategically poised to deliver differentiated therapeutics across the spectrum of cardiovascular thrombotic risk PONTE VEDRA, Fla., Sept. 15, 2025 (GLOBE NEWSWIRE) — Cadrenal Therapeutics, Inc. (Nasdaq: CVKD), a biopharmaceutical company developing transformative therapeutics to overcome the gaps in anticoagulation therapy, today announced the acquisition of the assets of eXIthera Pharmaceuticals (“eXIthera”), including its proprietary portfolio of investigational intravenous (IV) and oral Factor XIa inhibitors. The acquisition significantly enhances Cadrenal’s pipeline, adding drug candidates that address large and underserved segments of the current $38 billion global anticoagulation market. eXIthera’s lead asset, frunexian, is a first-in-class, Phase 2-ready intravenous (IV) Factor XIa inhibitor designed for acute care settings where contact activation of coagulation by medical devices plays a significant role, such as cardiopulmonary bypass, catheter thrombosis, and other blood-contacting implanted cardiac devices. The acquisition also includes EP-7327, an oral Factor XIa inhibitor, for the prevention and treatment of major thrombotic conditions. “With this acquisition, Cadrenal is the only company in the world developing a novel vitamin K antagonist (tecarfarin) and Factor XIa inhibitors, a promising new class of anticoagulants,” said Quang X. Pham, Chairman and CEO of Cadrenal Therapeutics. “These newly acquired assets will expand Cadrenal’s capabilities in an effort to address even more critical gaps in current antithrombotic treatment, especially for patients for whom current therapies are unreliable or carry excessive bleeding risk.” Unlike current anticoagulants on the market, which increase the risk of bleeding by broadly impairing coagulation, eXIthera’s compounds are mechanism-based inhibitors of Factor XIa, offering high potency, selectivity, and tunable pharmacokinetics. Factor XIa inhibition is one of the most active and exciting areas of current thrombosis research. “This acquisition reinforces Cadrenal’s long-term vision of becoming a category leader in anticoagulation,” added Pham. “With tecarfarin planning a trial in patients with end-stage kidney disease transitioning to dialysis, our plans for LVAD patients, and the current addition of frunexian and EP-7327, we believe that Cadrenal is strategically positioned to deliver differentiated therapeutics across the entire spectrum of patients with cardiovascular thrombotic risk.” Assets Acquired: Frunexian: Phase 2-ready IV Factor XIa agent for acute anticoagulationEP-7327: IND-ready oral small molecule candidate for chronic indicationsExtensive portfolio of additional novel FXIa small molecules Under a pre-existing license agreement, Sichuan Haisco Pharmaceuticals retains rights to frunexian in China, having completed a successful Phase 1 trial there. Under the terms of the license, Cadrenal will be entitled to receive royalties on future sales of frunexian in China. Deal Terms Overview: Under the terms of the acquisition agreement, eXIthera will receive milestone payments from Cadrenal totaling up to $15 million, contingent upon the realization of certain future clinical and regulatory milestones. Additionally, eXIthera will be entitled to royalties on global sales of the acquired assets upon future commercialization. The structure and terms of the agreement enable Cadrenal to focus capital deployment on advancing the clinical development of tecarfarin and the acquired assets. About Cadrenal Therapeutics, Inc.Cadrenal Therapeutics, Inc. is a biopharmaceutical company developing transformative therapeutics to overcome the gaps in anticoagulation therapy. Cadrenal’s lead investigational product is tecarfarin, a novel oral Vitamin K antagonist anticoagulant that is designed to address unmet needs in anticoagulation therapy. Tecarfarin is a reversible anticoagulant (blood thinner) designed to prevent heart attacks, strokes, and deaths due to blood clots in patients requiring chronic anticoagulation. Although warfarin is widely used, extensive clinical and real-world data have shown it can have significant, serious side effects. With tecarfarin, Cadrenal aims to reduce the clinical complexities of managing Vitamin K antagonists, particularly where direct-acting oral anticoagulants (DOACs) remain inadequate or unproven. Tecarfarin received Orphan Drug Designation (ODD) and fast-track designation for the prevention of systemic thromboembolism (blood clots) of cardiac origin in patients with end-stage kidney disease and atrial fibrillation (ESKD+AFib). The Company also received ODD for the prevention of thromboembolism and thrombosis in patients with implanted mechanical circulatory support devices, including Left Ventricular Assist Devices (LVADs). For more information, visit https://www.cadrenal.com/ and connect with the Company on LinkedIn. Safe Harbor Any statements in this press release about future expectations, plans, and prospects, as well as any other statements regarding matters that are not historical facts, may constitute “forward-looking statements.” The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potentially,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These statements include statements regarding Cadrenal’s ability to deliver differentiated therapeutics across the entire spectrum of cardiovascular thrombotic risk and overcome the gaps in anticoagulation therapy; the acquisition significantly enhancing Cadrenal’s pipeline and addressing large and underserved segments of the global anticoagulation market; the size of the global anticoagulation market; the potential of EP-7327 for the prevention and treatment of major thrombotic conditions; Cadrenal’s ability to address even more critical gaps in current antithrombotic treatment with the acquisition; Cadrenal becoming a leader in anticoagulation; commencement of a trial in patients with end-stage kidney disease transitioning to dialysis; Cadrenal’s receipt of royalties on future sales of frunexian in China; the payment to eXIthera of milestone payments by the Company totaling up to $15 million contingent upon the realization of certain future clinical and regulatory milestones as well as global sales of the acquired assets upon future commercialization; Cadrenal’s ability to focus capital deployment on advancing the clinical development of tecarfarin and the acquired assets; and tecarfarin addressing the unmet needs in anticoagulation therapy; tecarfarin reducing the clinical complexities of managing Vitamin K antagonists. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the Cadrenal’s ability to deliver differentiated therapeutics across the entire spectrum of cardiovascular thrombotic risk and overcome the gaps in anticoagulation therapy; the potential of EP-7327 for the prevention and treatment of major thrombotic conditions; Cadrenal’s ability to address even more critical gaps in current antithrombotic treatment; the payment of milestone payments and royalties; Cadrenal successfully advancing tecarfarin and the acquired assets into clinical practice; the commencement of a trial in patients with end-stage kidney disease transitioning to dialysis; tecarfarin addressing the unmet needs in anticoagulation therapy; tecarfarin reducing the clinical complexities of managing Vitamin K antagonists and the other risk factors described in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, and the Company’s subsequent filings with the Securities and Exchange Commission, including subsequent periodic reports on Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. Any forward-looking statements contained in this press release speak only as of the date hereof and, except as required by federal securities laws, the Company specifically disclaims any obligation to update any forward-looking statement, whether as a result of new information, future events, or otherwise. For more information, please contact: Cadrenal Therapeutics:Matthew Szot, CFOpress@cadrenal.com Investors:Lytham Partners, LLCRobert Blum, Managing Partner602-889-9700CVKD@lythampartners.com
Medtronic initiates global pivotal study of cardiac pacing in a new patient population
Study to evaluate whether a new approach to pacing the heart can improve the lives of patients with heart failure with preserved ejection fraction (HFpEF) who have limited treatment options today GALWAY, Ireland, Sept. 15, 2025 /PRNewswire/ — Medtronic plc (NYSE: MDT), a global leader in…