HOUSTON–(BUSINESS WIRE)–Saranas, a company focused on improving patient outcomes through early detection and monitoring of internal bleeding complications, announced today that the U.S. Department of Commerce’s United States Patent and Trademark Office (USPTO) has issued them Patent No. 11,224,414. Titled “Access Closure with Bleed Monitoring,” this key patent allows for embedding […]
Coronary/Structural Heart
Claritas Announces Receipt of Comments from Australian Ethics Committee Regarding the Company’s Phase 1 Clinical Study of R-107 and Closing of Financing
SAN FRANCISCO, CA and TORONTO, ON , Feb. 22, 2022 (GLOBE NEWSWIRE) — Claritas Pharmaceuticals, Inc. (TSX VENTURE: CLAS and OTC: KALTF) (the “Company” or “Claritas“) today announced that it has received comments from the Australian Human Research Ethics Committee (the “HREC”) regarding the Company’s Phase 1 clinical study of R-107, and […]
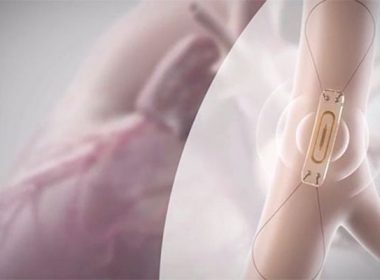
Abbott’s CardioMEMS™ HF System Receives FDA Approval to Support Patients Battling Earlier-Stage Heart Failure
– New expanded indication provides patients suffering from earlier stages of heart failure access to the CardioMEMS™ HF System, a small implantable sensor that can flag early warning signs of worsening heart failure – FDA approval was supported by data from the GUIDE-HF trial, which suggested that the CardioMEMS sensor […]
Breakthrough Study Uses Genetic Testing to Evaluate Risk of Heart Disease
PHOENIX, Feb. 17, 2022 /PRNewswire/ — Dignity Health in Arizona is launching the first research study in North America in which genetic testing is used to identify men and women at risk of developing heart disease based on the makeup of their DNA. If proven effective in clinical trials, this form of genetic testing may be adopted globally to prevent […]
Study Demonstrates Importance and Clinical Utility of Daxor’s Blood Volume (BVA-100®) Diagnostic in the Assessment of Heart Failure
Daxor’s BVA-100® Shown to Provide Unique and Actionable Data for Addressing Heart Failure Oak Ridge, TN, Feb. 17, 2022 (GLOBE NEWSWIRE) — Daxor Corporation (Nasdaq: DXR), the global leader in blood volume measurement technology, today announces new data demonstrating the importance and clinical utility of Daxor’s BVA-100 blood test in heart failure (HF) patients […]
Nuwellis Initiates REVERSE-HF Study to Evaluate Ultrafiltration for Heart Failure Patients with Fluid Overload
REVERSE-HF will evaluate rehospitalizations and survival when using the Aquadex therapy MINNEAPOLIS, Feb. 17, 2022 (GLOBE NEWSWIRE) — Nuwellis, Inc. (Nasdaq: NUWE) today announced the company will evaluate the clinical outcomes and economic value of its Aquadex® therapy in comparison to intravenous diuretics for the treatment of fluid overload in patients with worsening […]
Valbiotis Announces Approval to Launch the Two Phase II/III INSIGHT and INSIGHT 2 Clinical Studies, the Last Step in the Development of TOTUM•854 For the Reduction of Blood Pressure
These authorizations enable the simultaneous launch of recruitment for these international studies, which should be completed in the first half of 2023. The INSIGHT and INSIGHT 2 randomized, placebo-controlled studies are designed to include a total of 800 volunteers with mild to moderate elevated blood pressure, with 400 volunteers in […]
CLARIFY 2 AFFIRMS CLEERLY OVER INVASIVE HEART DISEASE EVALUATION METHODS
Findings from Cleerly’s groundbreaking CLARIFY 2 study echo the recent American College of Cardiology guidelines on the use of CCTA for non-invasive heart disease evaluation. The study, the second of six studies comparing Cleerly analysis against current gold standards to plaque and coronary artery imaging, demonstrates Cleerly’s effectiveness in the identification and exclusion of […]
Cytokinetics and the American Heart Association Bay Area Announce a Three-Year Collaboration to Advance Education and Awareness of Heart Disease
SOUTH SAN FRANCISCO, Calif. and OAKLAND, Calif., Feb. 16, 2022 (GLOBE NEWSWIRE) — Cytokinetics, Incorporated (Nasdaq: CYTK) and the American Heart Association (AHA) Bay Area today announced a three-year collaboration to accelerate education and awareness of heart disease. Within the collaboration, Cytokinetics will provide funding and support for several initiatives […]
LEXEO Therapeutics Announces FDA Clearance of Investigational New Drug Application for LX2006, an AAV-based Gene Therapy Candidate for Friedreich’s Ataxia Cardiomyopathy
– Phase 1/2 clinical trial expected to initiate in mid-2022 – – LX2006 is the first clinical-stage program from LEXEO’s cardiovascular pipeline and the third clinical-stage gene therapy candidate across its pipeline – NEW YORK, Feb. 16, 2022 (GLOBE NEWSWIRE) — LEXEO Therapeutics (LEXEO), a clinical-stage gene therapy company advancing a diverse […]