MedTech Startup Focused on Home Monitoring for Patients with Congestive Heart Failure and Other Chronic Diseases Wins the 2021 ICI Pitch Competition and $200,000 Award TEL AVIV, Israel, Dec. 8, 2021 /PRNewswire/ — Donisi Health (Donisi) prevailed in this year’s ICI Innovation Competition – the premier international conference for innovations in cardiovascular intervention. Competing against […]
Coronary/Structural Heart
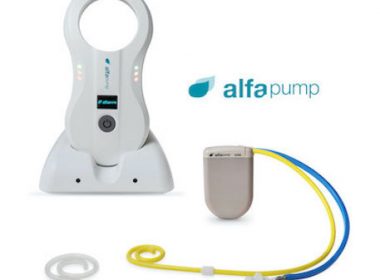
Sequana Medical announces positive interim results of SAHARA DESERT, the alfapump DSR® study in heart failure patients with persistent congestion
Interim data from six patients indicate that alfapump DSR therapy can: safely, effectively and rapidly eliminate persistent congestion and restore euvolemia in diuretic-resistant heart failure patients, considerably benefit cardio-renal status, and dramatically improve diuretic responsiveness for months post–treatment Recruitment on–track to report top–line data in H2 2022 Long-term follow-up of RED DESERT patients shows durable improvement in diuretic response following alfapump DSR therapy Proprietary DSR Infusate 2.0 development on track to start MOJAVE […]
Foldax Receives Approval for Clinical Trial of TRIA Biopolymer Surgical Aortic Heart Valve in India
SALT LAKE CITY–(BUSINESS WIRE)–Foldax®, Inc. today announced that the Drugs Controller General of India (DCGI), India’s regulatory body for medical devices, has approved initiation of a clinical trial of the TRIA™ biopolymer surgical aortic heart valve. The TRIA valve reimagines the heart valve by combining the company’s proprietary biopolymer – […]
Restore Medical Announces First in Human Trial for Treatment of Heart Failure in Patients
ContraBand™ technology helps restore the heart’s functionality and may significantly improve patients’ quality of life OR YEHUDA, Israel, Dec. 7, 2021 /PRNewswire/ — Restore Medical Ltd., a clinical-stage MedTech company developing cardiac implants for the treatment of congestive heart failure (CHF), today announced promising preliminary results of the First-in-Human clinical trial of ContraBand™, a breakthrough treatment […]
Saranas® Announces Initial Enrollment of Clinical Trial Assessing the Utility of Early Bleed Detection in Patients Undergoing Mechanical Circulatory Support
SAFE-MCS Will Examine the Impact of the Early Bird® Bleed Monitoring System on Reducing Bleeding Complications in Patients Undergoing High Risk PCI with Mechanical Circulatory Support HOUSTON–(BUSINESS WIRE)–Saranas, Inc., has announced initiation and first patient enrollment in SAFE-MCS, a multi-center, single arm, open-label clinical trial evaluating the safety of complex high-risk […]
Olink participates in one of the largest proteomics studies enabling new treatment options for patients with heart failure
UPPSALA, Sweden, Dec. 02, 2021 (GLOBE NEWSWIRE) — Olink Holding AB (publ) (“Olink”) (Nasdaq: OLK), today announced that its project with Boehringer Ingelheim, assessing the potential utility of protein biomarkers in the empagliflozin EMPEROR studies, recently entered the data analysis phase. Empagliflozin, approved in the US under the tradename JARDIANCE, […]
Sugar molecule flushes away cholesterol and reverses blocked arteries
Gold Coast, Queensland, Australia, Dec. 02, 2021 (GLOBE NEWSWIRE) — Cholrem (Australia) Pty Ltd have produced a product CAVADEX (Beta-Cyclodextrin) which reverses heart disease by removing cholesterol from damaged and narrowed arteries after only a few months, resulting in reduced arterial plaque. Atherosclerosis, also known as heart disease, is the […]
PhaseBio Announces Publication of Interim Results from Pivotal REVERSE-IT Phase 3 Trial of Bentracimab in NEJM Evidence
Results previously presented in a Late-Breaking Science Session at the American Heart Association’s 2021 Scientific Sessions Global Phase 3 trial of bentracimab achieved both primary reversal endpoint and co-primary endpoint of clinical hemostasis Bentracimab had no drug-related serious adverse events MALVERN, Pa. & SAN DIEGO–(BUSINESS WIRE)–PhaseBio Pharmaceuticals, Inc. (Nasdaq: PHAS), a […]
Robocath successfully completes first robotic coronary angioplasty with R‑One in China
Rouen, France, November 30, 2021 – Robocath, a company that designs, develops and commercializes innovative robotic platforms for the treatment of vascular diseases, today announces the successful completion of the first coronary angioplasty in China assisted by its R-One™ robotic platform. The procedure took place at the 301 Hospital in Beijing on […]
Neovasc Reducer™ System Receives National Reimbursement in France
First therapy to receive newly established French reimbursement designation VANCOUVER and MINNEAPOLIS, Nov. 30, 2021 (GLOBE NEWSWIRE) — via NewMediaWire — Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ, TSX: NVCN) today announced that the Neovasc Reducer™ (“Reducer”) system has been granted Prise en Charge Transitoire (“PECT”) reimbursement in France by the national health authority, Haute Autorité de Santé (“HAS”). The decision […]