ST. PAUL, Minn.–(BUSINESS WIRE)–Cardiovascular Systems, Inc. (CSI®) (NASDAQ: CSII), a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, announced today the start of enrollment in a first in-human trial of the coronary everolimus drug-coated balloon (DCB) being developed by Chansu […]
Coronary/Structural Heart
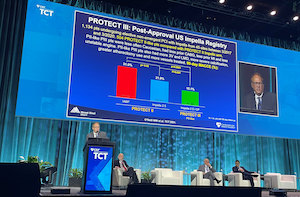
Two Large Studies Demonstrate Complete Revascularization with Impella Heart Pumps Improves Ejection Fraction and Long-Term Patient Outcomes
ORLANDO, Fla.–(BUSINESS WIRE)–The final results of the PROTECT III and Restore EF prospective studies demonstrate improved outcomes for high-risk PCI patients with the use of Impella heart pumps. The study results were reviewed this morning at Transcatheter Cardiovascular Therapeutics (TCT) 2021, the annual scientific symposium of the Cardiovascular Research Foundation, by Gregg […]
Medtronic study shows patients with high blood pressure are interested in an interventional procedure treatment option
New data from Medtronic Patient Preference study presented at TCT 2021; Medtronic adds to its clinical leadership for renal denervation with launch of the SPYRAL AFFIRM study DUBLIN and ORLANDO, Fla., Nov. 4, 2021 /PRNewswire/ — Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced findings from a new study of the Patient […]
PLx Pharma Inc. Announces New Scientific Data Available Evaluating the Pharmacologic Profile of FDA-Approved VAZALORE 81 mg Liquid-Filled Aspirin Capsules
— VAZALORE 81 mg shows fast bioavailability, absorption, and platelet inhibition — — VAZALORE 81 mg has pharmacokinetic and pharmacodynamic profiles consistent with VAZALORE 325 mg — — Findings presented at TCT meeting earlier today — SPARTA, N.J., Nov. 04, 2021 (GLOBE NEWSWIRE) — PLx Pharma Inc. (NASDAQ: PLXP) (“PLx” […]
Retrospective Analysis Using the VoluMetrix NIVA|HF Investigational Algorithm to Assess Patients With Heart Failure Predicts Hospitalization
The NIVA|HF investigational algorithm uses proprietary venous waveform technology from VoluMetrix to assess otherwise hard-to-obtain cardiovascular metrics. According to study authors, ‘This non-invasive measurement has potential for … prevention of hospital admission in heart failure patients.’ NASHVILLE, Tenn., Nov. 04, 2021 (GLOBE NEWSWIRE) — VoluMetrix, a Nashville-based biotech startup dedicated […]
ReCor Medical Reports Latest Data from Landmark Renal Denervation Clinical Trials at TCT Annual Meeting
RADIANCE-HTN TRIO and SOLO trial updates demonstrate the durability of blood pressure lowering effects of the Paradise Ultrasound Renal Denervation System in a broad spectrum of patients with hypertension. PALO ALTO, Calif.–(BUSINESS WIRE)–Otsuka Medical Devices Co., Ltd. (“Otsuka Medical Devices”), a fully owned subsidiary of Otsuka Holdings Co., Ltd., and […]
Colibri Heart Valve Initiates International CE Mark-enabling Pilot Study of Second-Generation TAVI System
LOUISVILLE, CO , Nov. 03, 2021 (GLOBE NEWSWIRE) — Colibri Heart Valve LLC, a privately held emerging medical device company, today announced the initiation of an international pilot study of the Colibri transcatheter aortic valve implantation (TAVI) system. Bernard Chevalier, M.D., an interventional cardiologist at Hôpital Privé Jacques Cartier, is serving […]
Sequana Medical receives MDSAP certification and expands its Quality Management System towards North America
Ghent, Belgium – 3 November 2021 – Sequana Medical NV (Euronext Brussels: SEQUA, the “Company”), an innovator in the treatment of diuretic-resistant fluid overload in liver disease, malignant ascites and heart failure, today announces it has received Medical Device Single Audit Program (MDSAP) certification from its auditing organisation British Standards Institution (BSI), thereby expanding its […]
First U.S. Patient Enrolled in Cardionomic’s CPNS Pilot Study
Study evaluates safety and performance of endovascular pulmonary nerve stimulation in ADHF MINNEAPOLIS, Nov. 2, 2021 /PRNewswire/ — Cardionomic, Inc., a Minneapolis medical device company, is pleased to announce initial U.S. enrollment in their global Cardiac Pulmonary Nerve Stimulation (CPNS) Pilot Study (NCT04814134). The study is evaluating the safety and performance of the CPNS System […]
Ionis initiates pivotal Phase 3 clinical study of olezarsen in patients with severe hypertriglyceridemia
– More than 3 million people in the US have severe hypertriglyceridemia – The CORE Phase 3 clinical study further expands Ionis’ late-stage pipeline and is the second Phase 3 clinical study in Ionis’ broad olezarsen development program – Olezarsen is one of Ionis’ wholly owned medicines the company plans […]