Commercial Launch Follows Successful Beta Testing of Prevencio’s Highly Accurate AI-driven HART Tests KIRKLAND, Wash.–(BUSINESS WIRE)–Prevencio, Inc. today announces an Agreement with Atlas Genomics to commercially launch its highly accurate, AI-driven HART blood tests for heart disease and risk of heart attack, stroke, and cardiovascular death. The HART blood tests are […]
Coronary/Structural Heart
BioStable Science & Engineering Announces CE Mark Approval for the HAART 200 Aortic Annuloplasty Device & Surpasses 1,000 Patients Treated Worldwide with the HAART Devices
AUSTIN, Texas–(BUSINESS WIRE)–BioStable Science & Engineering, Inc. (“BioStable”) announced today the company has received CE Mark approval for the HAART 200 Aortic Annuloplasty Device for use during bicuspid aortic valve repair. With CE Mark approval of both the HAART 300 and HAART 200 Aortic Annuloplasty Devices, BioStable will be able to […]
Vivasure Medical Begins Clinical Evaluation of Next-Generation PerQseal+ Device
First patient enrolled in Frontier V study evaluating enhanced fully absorbable, patch-based large-bore percutaneous closure device GALWAY, Ireland–(BUSINESS WIRE)–Vivasure Medical®, a company pioneering novel fully absorbable technology for percutaneous vessel closure, today announced the first patient was enrolled in the Frontier V study, a European multicenter study evaluating PerQseal®+, the […]
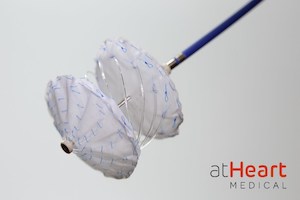
Newly Formed Company, atHeart Medical, Aims to Evolve Septal Closure with the reSept™ ASD Occluder
Company prepares for U.S. IDE clinical trial of its novel metal-free frame designed to reduce complications and preserve long-term treatment options BAAR, Switzerland and MOUNTAIN VIEW, Calif., April 20, 2021 /PRNewswire/ — atHeart Medical, a medical device company dedicated to establishing the new standard of care for closure of atrial septal defects (ASD), announced today that […]
MyoStrain® Provides Comprehensive Assessment of Myocardial Deformation to Help Physicians Risk-Stratify Heart Failure Patients for Individualized Cardiac Treatment
MyoStrain provided a uniquely sensitive and reproducible measure of segmental heart function to help physicians quantify differences in heart failure patients and risk-stratify patient populations based on the individual extent and severity of dysfunction MORRISVILLE, N.C., April 19, 2021 /PRNewswire/ — A new article published in the European Society of Cardiology (ESC) Heart […]
Quantum Genomics Announces the End of its Collaboration with Qilu for the Development and Commercialization of Firibastat in China
PARIS and NEW YORK, April 20, 2021 (GLOBE NEWSWIRE) — Quantum Genomics (Euronext Growth – FR0011648971 – ALQGC), a biopharmaceutical company specializing in developing a new drug class that directly targets the brain to treat difficult-to-treat and resistant hypertension and heart failure, announces the end of its collaboration with Chinese Pharma […]
SANUWAVE® Rebrands to Reflect Evolved Wound Care Solutions
“Energy First” Protocol Improves Clinical Outcomes SUWANEE, Ga.–(BUSINESS WIRE)–SANUWAVE Health, Inc. (OTCQB: SNWV), focused on the development and commercialization of a robust and innovative advanced wound care product portfolio for the repair and regeneration of skin and vascular structures, today announced its rebranding. The new brand reflects the evolution of […]
LivaNova Receives FDA 510(k) Clearance for B-Capta, the New In-Line, Blood-Gas Monitoring System Integrated Into the S5 Heart-Lung Machine
Intuitive, optical-based technology system now available around the world LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology and innovation company, today announced it received U.S. Food and Drug Administration (FDA) 510(k) clearance for B-Capta®, the new in-line, blood-gas monitoring system integrated into the market-leading S5® heart-lung machine (HLM). The system is […]
U.S. FDA Approves CytoSorbents to Initiate U.S. STAR-T Trial For Ticagrelor Removal During Cardiothoracic Surgery
MONMOUTH JUNCTION, N.J., April 19, 2021 /PRNewswire/ — CytoSorbents Corporation (NASDAQ: CTSO), a critical care leader whose flagship E.U. approved CytoSorb® blood purification technology is intended to treat deadly conditions in critically-ill and cardiac surgery patients, announces that the U.S. Food and Drug Administration (FDA) has granted conditional approval of its investigational device exemption (IDE) application for […]
Windtree Therapeutics Announces New Istaroxime Expedited Review Filing of Patent
Company Continues to Leverage Data to Strengthen the Istaroxime Patent Estate WARRINGTON, Pa., April 19, 2021 /PRNewswire/ — Windtree Therapeutics, Inc. (NasdaqCM: WINT) (“Windtree” or the “Company”), a biotechnology and medical device company focused on advancing multiple late-stage interventions for acute cardiovascular and pulmonary disorders, today announced it has filed a Track One […]