Filing includes one year-follow up data from the STTAR Clinical Study. NEWTOWN, Pa., Jan. 28, 2021 /PRNewswire/ — Micro Interventional Devices, Inc.™ (MID) announced today that it has submitted the required technical documentation for CE Mark approval for its MIA-T™ Percutaneous Tricuspid Annuloplasty System for tricuspid valve repair to its Notified Body. The receipt of a […]
Coronary/Structural Heart
Medicure Announces Early Completion of Enrollment for iSPASM, a Phase 1/2a Exploratory Clinical Trial of AGGRASTAT® (tirofiban hydrochloride) Injection vs. Placebo for Induced Suppression of Platelets Activity in Aneurysmal Subarachnoid Hemorrhage Management
WINNIPEG, MB, Jan. 27, 2021 /PRNewswire/ – Medicure Inc. (“Medicure” or the “Company”) (TSXV: MPH) (OTC: MCUJF), a cardiovascular pharmaceutical company, is pleased to announce the early completion of iSPASM, a randomized, double-blind, single-center, Phase 1/2a trial aimed at assessing the safety of long-term (7-day) use of AGGRASTAT® (tirofiban hydrochloride) injection (an intravenous GP IIb/IIIa […]
OM1 Heart Failure Registry Reaches More than 140,000 Patients Prospectively Followed with Deep Clinical Data
Available for collaborations, analytics, and licensing for faster, more cost-effective access to research-grade heart failure data BOSTON, Jan. 28, 2021 /PRNewswire/ — OM1, a leading real-world data (RWD), outcomes and technology company with a focus on chronic diseases, today announced the launch of an expanded Heart Failure registry. Heart Failure (HF) is considered […]
PhaseBio Announces Dosing of First Patient in European Union as Part of REVERSE-IT Global Phase 3 Trial of Bentracimab for Reversal of Antiplatelet Effects of Ticagrelor
Cardiovascular disease remains a leading cause of mortality in both the European Union and globally, with antiplatelet therapy being a core treatment to prevent cardiovascular events The lack of reversal agents for patients taking antiplatelet therapies who require urgent surgery or experience a major bleeding event remains a critical unmet need […]
CHF Solutions’ Physician-Led Webinar Highlights Real-World Evidence Supporting Effectiveness of Ultrafiltration in Heart Failure Patients
EDEN PRAIRIE, Minn., Jan. 28, 2021 (GLOBE NEWSWIRE) — CHF Solutions (Nasdaq: CHFS), a medical device company dedicated to changing the lives of patients suffering from fluid overload, today announced key takeaways from its physician-led webinar discussing real-world experience using ultrafiltration (UF) in the management of heart failure patients. Panelists […]
Cardio Diagnostics Launches Epi+Gen CHD™, A New Heart Disease Risk Assessment Test
On Average, 41% More Sensitive Than Current Tests for Predicting Future Heart Disease in Women CHICAGO–(BUSINESS WIRE)–Cardio Diagnostics, Inc, a biotechnology company making cardiovascular disease prevention and early detection more accessible, personalized and precise, announces the launch of its patent-pending flagship product, Epi+Gen CHD. The Epi+Gen CHD test sensitively assesses […]
PhaseBio Presents Data from Phase 1b/2a Trial of Pemziviptadil for the Treatment of Pulmonary Arterial Hypertension at 15th Pulmonary Vascular Research Institute Virtual World Congress
New data for pemziviptadil (PB1046) support continued evaluation as a potential novel therapy of once-weekly VIP analogue for adults with pulmonary arterial hypertension; novel agent observed to be well tolerated, with no drug-related serious adverse events resulting in study drug discontinuation MALVERN, Pa. & SAN DIEGO–(BUSINESS WIRE)–PhaseBio Pharmaceuticals, Inc. (Nasdaq: PHAS), a […]
Ancora Heart Enrolls First Patient in the CORCINCH-HF Study
Pivotal Trial to Evaluate Safety and Efficacy of AccuCinch® Ventricular Restoration System in Heart Failure Patients with Reduced Ejection Fraction SANTA CLARA, Calif.–(BUSINESS WIRE)–Ancora Heart, Inc., a company developing a novel therapy to address heart failure, today announced enrollment of the first patient in the CORCINCH-HF pivotal trial, which is designed […]
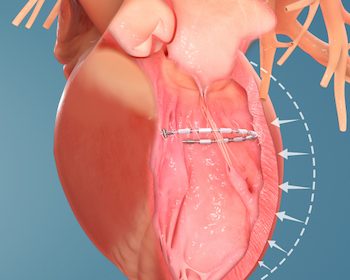
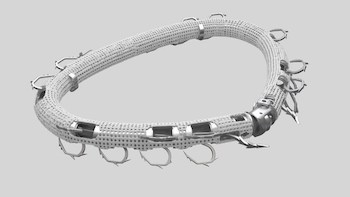
Valcare Medical Announces First-in-Human Transseptal Implant of the AMEND™ Annuloplasty Ring for Mitral Valve Repair
HERZLIYA, Israel, Jan. 27, 2021 /PRNewswire/ — Valcare Medical Ltd. developer of transcatheter mitral and tricuspid valve repair and replacement solutions, announces that it has successfully completed its first-in-human transseptal delivery of the AMEND™ annuloplasty ring. The transseptal AMEND procedure was performed at the Schulich Heart Centre at Sunnybrook Health Sciences Centre in Toronto, under […]
Microbot Medical Announces Successful Outcome of its Discussions with FDA Regarding Regulation of Self-Cleaning Shunt
FDA Feedback Reflects Strength of Pre-Clinical Safety Data; Maintains Q3 2022 Projected Commencement of First-in-Human Clinical Trial HINGHAM, Mass., Jan. 27, 2021 (GLOBE NEWSWIRE) — Microbot Medical Inc. (Nasdaq: MBOT) announced the completion of successful discussions with the U.S. Food and Drug Administration (FDA) for its Self-Cleaning Shunt (SCS). After review […]