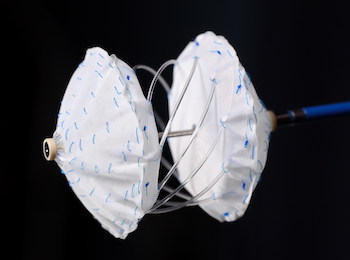
New data on successful transplantations for high-risk profile donor hearts presented at the American Transplant Congress CAMBRIDGE, Mass.–(BUSINESS WIRE)–Paragonix Technologies, Inc. announced the presentation of new clinical data at the American Transplant Congress (ATC) on the successful use of the Paragonix SherpaPak™ Cardiac Transport System (CTS) for high-risk donor hearts at the […]
Coronary/Structural Heart
SARS-CoV-2 Cell Entry Mechanisms in Intact Human Heart Published in JACC: Basic to Translational Science by University of Colorado Anschutz Medical Campus and ARCA biopharma Investigators
Integrin A5 may be a new target for intervening in the cell infectious process Findings may lead to the development of precision therapeutic approaches to prevent SARS-CoV-2-cell entry while preserving the functional activity of ACE2 WESTMINSTER and AURORA, Colo., July 07, 2020 (GLOBE NEWSWIRE) — The University of Colorado Anschutz Medical […]
Celixir provides update on ongoing clinical programme with lead therapeutic candidate CLXR-001
Celixir provides update on ongoing clinical programme with lead therapeutic candidate CLXR-001 First patient successfully dosed in cardiomyopathy study Stratford-upon-Avon, UK: July, 6 2019 – Celixir, a privately-owned company discovering and developing life-saving advanced therapies, announces an update to its ongoing clinical programme with lead therapeutic candidate CLXR-001. The first patient was […]
Carag Receives U.S. FDA IDE Approval to Conduct Clinical Study of First Transcatheter Septal Occluder With Bioresorbable, Metal-free Framework
The U.S. trial for CE-marked CBSO is designed to enroll up to 250 patients in a staged study approach BAAR, Switzerland, July 1, 2020 /PRNewswire/ — CARAG AG, a privately-held Swiss medical device development company, today announced receiving U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) approval for its Carag Bioresorbable […]
Berlin Heart Completes Post Approval Surveillance; Report Details Improved Outcomes for Pediatric Heart Failure Patients Supported with EXCOR® Pediatric
THE WOODLANDS, Texas–(BUSINESS WIRE)–Berlin Heart Inc. announced they have completed the Post Approval Surveillance for the EXCOR® Pediatric Ventricular Assist Device, a requirement of the Post Market Approval (PMA) granted by the Food and Drug Administration (FDA), in June 2017. The final report submitted to the FDA confirmed the positive contemporary […]
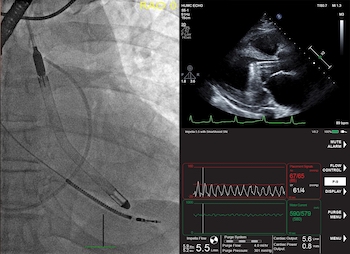
Study Finds 84% Survival Rate in Patients in Cardiogenic Shock and Other Challenging Cardiac Conditions with the New Impella 5.5 with SmartAssist
DANVERS, Mass.–(BUSINESS WIRE)–The first published United States experience of patients who received Abiomed’s newest heart pump, Impella 5.5 with SmartAssist, finds 84% of the patients survived to explant with 76% native heart recovery. The study was published in the July edition of the American Society for Artificial Internal Organs (ASAIO) Journal. The study examined […]
Esperion Announces Publication in the Journal of the American Medical Association Cardiology of Pooled Efficacy Analysis from the Phase 3 LDL-C Lowering Clinical Development Program of NEXLETOL® (bempedoic acid) Tablets
ANN ARBOR, Mich., July 01, 2020 (GLOBE NEWSWIRE) — Esperion (NASDAQ: ESPR) today announced that pooled efficacy analysis from the four Phase 3 clinical studies of NEXLETOL, an oral, once-daily LDL-cholesterol lowering medicine, was published in the Journal of the American Medical Association (JAMA) Cardiology. The four Phase 3 clinical studies […]
Matinas BioPharma Resumes Enrollment in the ENHANCE-IT and EnACT Clinical Trials
– Topline data from ENHANCE-IT study of MAT9001 vs. Vascepa® expected in Q1 2021 – – EnACT remains on track for cohort progression in Q4 2020 – BEDMINSTER, N.J., June 30, 2020 (GLOBE NEWSWIRE) — Matinas BioPharma Holdings, Inc. (NYSE AMER: MTNB), today announced that it has commenced enrollment and started dosing […]
BioCardia Announces Activation of Pivotal Trial Studying CardiAMP Cell Therapy Trial to Treat Chronic Myocardial Ischemia
First Site Initiation Visit in Trial Completed and Executive Steering Committee Named SAN CARLOS, Calif., July 01, 2020 (GLOBE NEWSWIRE) — BioCardia®, Inc. [NASDAQ:BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced activation of a pivotal trial studying the Company’s investigational CardiAMP® cell therapy in the treatment of […]
scPharmaceuticals Announces FUROSCIX® NDA Resubmission
BURLINGTON, Mass.–(BUSINESS WIRE)–scPharmaceuticals Inc. (Nasdaq: SCPH), a pharmaceutical company focused on developing and commercializing products that have the potential to optimize the delivery of infused therapies, advance patient care, and reduce healthcare costs, today announced that it has resubmitted its 505(b)(2) New Drug Application (NDA) to the U.S. Food and […]