SAN DIEGO, June 10, 2025 (GLOBE NEWSWIRE) — Gyre Therapeutics (“Gyre”) (Nasdaq: GYRE), an innovative, commercial-stage biopharmaceutical company dedicated to advancing fibrosis-first therapies across organ systems affected by chronic disease, today announced that the first volunteer has been successfully dosed in a Phase 1 clinical trial evaluating F230, a novel endothelin A (“ETA”) receptor antagonist, for the treatment of pulmonary arterial hypertension (“PAH”).This milestone marks Gyre’s entry into the PAH field, a rare, progressive, and high-mortality cardiovascular condition with limited treatment options. PAH is recognized in China’s National Rare Disease Catalog, underscoring its significance in public health. According to Frost & Sullivan, China’s PAH market was valued at $370 million in 2023 and is projected to grow to $480 million by 2031.F230, originally discovered by Eisai Co., Ltd. and exclusively licensed by GNI Group Ltd. to Gyre, is a fully synthetic small molecule designed to selectively block the ETA receptor. By targeting this pathway, F230 is designed to reduce pulmonary vascular remodeling and lower pulmonary pressure, key contributors to PAH progression.The Phase 1 trial is designed to evaluate safety, tolerability, and pharmacokinetics in healthy volunteers. The trial represents the latest expansion of Gyre’s fibrosis-first strategy beyond the liver, leveraging a robust clinical development platform and commercial infrastructure in China.F230 joins Gyre’s pipeline alongside lead candidate Hydronidone (F351), which met the primary endpoint in a pivotal Phase 3 trial for CHB-fibrosis. A New Drug Application (“NDA”) submission to China’s National Medical Products Administration (“NMPA”) is planned for the third quarter of 2025, and a pre-IND meeting with the U.S. Food and Drug Administration is being planned for an expected Phase 2 trial in metabolic dysfunction-associated steatohepatitis (“MASH”) fibrosis.About Gyre TherapeuticsGyre Therapeutics is a biopharmaceutical company headquartered in San Diego, CA, primarily focused on the development and commercialization of Hydronidone for liver fibrosis, including MASH, in the U.S. Gyre’s strategy builds on its experience in mechanistic studies using MASH rodent models and clinical studies in CHB-induced liver fibrosis. In the People’s Republic of China, Gyre is advancing a broad pipeline through its indirect controlling interest in Gyre Pharmaceuticals, including therapeutic expansions of ETUARY, and development programs for F573, F528, and F230.Forward-Looking StatementsThis press release contains “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, which statements are subject to substantial risks and uncertainties and are based on estimates and assumptions. All statements, other than statements of historical facts included in this press release, are forward-looking statements, including statements concerning the expectations regarding Gyre’s research and development efforts and timing of expected clinical trials, including an NDA submission to the NMPA for F351, the expected clinical benefits of F230 and expectations regarding interactions with regulators. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “plan” or the negative of these terms, and similar expressions intended to identify forward-looking statements. These statements reflect our plans, estimates, and expectations, as of the date of this press release. These statements involve known and unknown risks, uncertainties and other factors that could cause our actual results to differ materially from the forward-looking statements expressed or implied in this press release. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation: Gyre’s ability to execute on its clinical development strategies; positive results from a clinical trial may not necessarily be predictive of the results of future or ongoing clinical trials; the timing or likelihood of regulatory filings and approvals; competition from competing products; the impact of general economic, health, industrial or political conditions in the United States or internationally; the sufficiency of Gyre’s capital resources and its ability to raise additional capital. Additional risks and factors are identified under “Risk Factors” in Gyre’s Annual Report on Form 10-K for the year ended December 31, 2024 filed on March 17, 2025 and in other filings Gyre may make with the SEC. Gyre expressly disclaims any obligation to update any forward-looking statements whether as a result of new information, future events or otherwise, except as required by law.Investor Contact:David ZhangGyre Therapeuticsdavid.zhang@gyretx.com
Coronary/Structural Heart
Nuevocor Announces FDA Clearance of IND for NVC-001 for LMNA-Related Dilated Cardiomyopathy
LMNA-related dilated cardiomyopathy (LMNA DCM) is one of the most aggressive forms of DCM, affecting approximately 100,000 individuals in the United States and Europe, who progress rapidly to end-stage heart failure. NVC-001 demonstrated significant benefits, including survival and…
Merck Announces Positive Topline Results From the First Two Phase 3 CORALreef Trials Evaluating Enlicitide Decanoate for the Treatment of Adults With Hyperlipidemia
Enlicitide demonstrated statistically significant and clinically meaningful reductions in LDL-C in both Phase 3 CORALreef HeFH and CORALreef AddOn trials Enlicitide, a novel macrocyclic peptide, has the potential to be the first approved oral PCSK9 inhibitor RAHWAY, N.J.–(BUSINESS WIRE)–Merck (NYSE: MRK), known as MSD outside of the United States and […]
Microtech Announces the First U.S. Atrial Microsensor Implantations as Part of its FIH Study
TEL AVIV, Israel, June 9, 2025 /PRNewswire/ — Microtech, a wholly owned subsidiary of Medinol, is happy to announce the first U.S. implantations of its atrial-pressure microsensor. Two surgical implantations were performed on Friday, May 16, 2025, at New York-Presbyterian/Columbia…
Pepin Heart Institute at AdventHealth Tampa becomes first in Tampa Bay to complete 1,000th WATCHMAN™ heart procedure
TAMPA, Fla., June 6, 2025 /PRNewswire/ — The heart care experts at the Pepin Heart Institute, a part of AdventHealth Tampa, are now the first in the Tampa Bay area to complete 1,000 WATCHMAN ™ procedures, a minimally-invasive surgery that helps prevent stroke and cardiovascular death in…
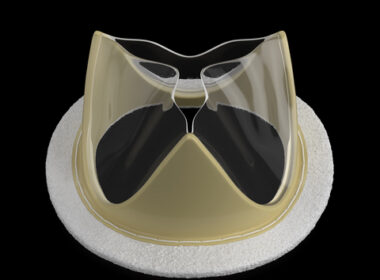
Foldax Secures Approval for TRIA Mitral Heart Valve in India
SALT LAKE CITY–(BUSINESS WIRE)–Foldax® Inc., a leader in heart valve innovation, today announced that the Indian Central Drugs Standard Control Organization (CDSCO) approved its TRIA™ Mitral Valve. Dolphin Life Science India LLP will locally manufacture the TRIA Mitral Valve in India. Foldax’s vision for its novel polymer heart valves is […]
FineHeart Receives ANSM1 Authorization to Deploy Its First-In-Human Clinical Study in France
Patients with advanced heart failure will be treated with FlowMaker®, an innovative, fully implantable device for restoring cardiac output. BORDEAUX, France–(BUSINESS WIRE)–FineHeart S.A., a clinical-stage medical technology company specializing in the development of innovative solutions for cardiology, announces that it has received authorization from the French National Agency for the […]
MemorialCare Heart and Vascular Institute Completes First Successful TriClip Procedure for Tricuspid Valve Repair
LONG BEACH, Calif., June 4, 2025 /PRNewswire/ — The cardiology team at the MemorialCare Heart & Vascular Institute at Long Beach Medical Center, led by Chief of Cardiology David Shavelle, M.D., performed Long Beach Medical Center’s first transcatheter tricuspid valve replacement…
Vasa Therapeutics Announces Successful Completion of First-in-Human Study for Investigational Therapy VS-041, a Small Molecule Drug Candidate for Heart Failure with Preserved Ejection Fracture
VS-041 safe and well tolerated in healthy patients Biomarker trial in patients with HFpEF targeted to initiate in 2025 ENCINITAS, Calif. and WROCLAW, Poland, June 4, 2025 /PRNewswire/ — Vasa Therapeutics (“Vasa”), a clinical stage biopharmaceutical company developing novel therapies for…
FastWave Medical Announces Successful First-in-Human Procedures in Study of Sola™, Its Next-Generation Coronary Laser IVL System
Initial FIH procedures launch FastWave’s multi-center feasibility study evaluating the safety and performance of its laser-based coronary IVL platform. MINNEAPOLIS, June 3, 2025 /PRNewswire/ — FastWave Medical, a pioneering intravascular lithotripsy (IVL) startup, today announced the…