SEATTLE, July 16, 2024 (GLOBE NEWSWIRE) — Faraday Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company focused on the prevention of heart failure by reducing myocardial damage following acute ST-elevation myocardial infarction (STEMI) through the reduction of reperfusion injury during percutaneous coronary intervention (PCI), today announced it will host a virtual KOL webinar on Tuesday, July 30, 2024, at 11:00 AM ET. To register for the webinar, click here. The webinar will feature Dr. Cheerag Shirodaria (Oxford University Hospitals), Dr. Marc Bonaca (University of Colorado Anschutz), and Dr. Antonio Gutierrez (Duke University Hospital and Durham VA Medical Center), who will discuss the unmet need and current treatment landscape in preventing ischemia-reperfusion injury and highlight the effects of STEMI on heart failure and other patient outcomes. The webinar will also review data from the development of FDY-5301, an investigational product being evaluated by Faraday in minimizing cardiac damage following acute STEMI. The company’s pivotal Phase 3 Iocyte AMI-3 trial of FDY-5301 in anterior STEMI patients undergoing primary percutaneous intervention is now fully enrolled with topline data anticipated in 2H 2025. A live question and answer session will follow the formal presentations. About Cheerag Shirodaria, BSc, MBBS, MD, FRCP, MBA Dr. Cheerag Shirodaria is Chief Development Officer at Caristo Diagnostics and a Cardiologist at Oxford University Hospitals in the UK. He is an interventional cardiologist who is also an expert in cardiac imaging, particularly cardiac MRI (CMR) and coronary CT angiography (CCTA), having completed his doctoral thesis in Oxford on novel vascular imaging techniques using CMR. He was heavily involved in the design and execution of the phase 2 STEMI trial involving FDY-5301 and is a member of the Executive Steering Committee of the IOCYTE-AMI trial. As co-founder and former CEO of Caristo, he has developed and commercialized novel imaging AI imaging tools to predict future cardiometabolic risk. Dr Shirodaria obtained his medical degree from St Bartholomew’s Hospital Medical College, his medical doctorate from the University of Oxford and his MBA from London Business School. About Marc Bonaca, MD, MPH Dr. Marc Bonaca is a cardiologist and vascular medicine specialist serving as executive director of CPC and Director of Vascular Research & Professor of Medicine at the University of Colorado Anschutz. Dr. Bonaca earned his medical degree from the University of Connecticut and his MPH at Harvard. He completed his medical residency, cardiology fellowship, and vascular medicine fellowship at Brigham and Women’s Hospital and a dedicated research fellowship at the TIMI Study Group. He became faculty at BWH and Harvard Medical School and an investigator at TIMI. He directed pharmacovigilance at TIMI and was an investigator on several large outcomes trials including TRA2P-TIMI 50, PEGASUS-TIMI 54, DECLARE-TIMI 58, and REAL TIMI 63B. In 2018, he joined the faculty at the University of Colorado as Professor of Medicine and the William R. Hiatt Endowed Chair in Cardiovascular Research. He is the Executive Director of CPC, an affiliated non-profit Academic Research Organization. At CPC, he has led several clinical trials with CPC as the lead ARO, including VOYAGER PAD, PREVENT HD, and ASPEN, and is leading several ongoing trials, including BRIGHT and EVOLVE MI, a 4000-patient pragmatic multinational acute coronary syndrome trial. Dr. Bonaca’s research focus is on ischemic risk with atherosclerotic vascular disease, risk prediction, and risk modification using pharmacologic and biologic therapies. He has extensive experience designing and conducting large, multicenter randomized clinical trials, and analyzing registries and real-world datasets. His areas of interest include PAD, PVD, and diabetes, with a focus on the breadth of risk including ischemic limb outcomes, microvascular complications, and major adverse cardiovascular events. He is also investigating the cardiac, vascular, and thrombotic complications associated with novel oncologic therapies. At CPC, he has focused on building a robust faculty and operational group dedicated to high quality, efficient trials leveraging health networks, informatics, and decentralized design. About J. Antonio T. Gutierrez, MD Dr. Antonio Gutierrez’s clinical interests include diagnostic and interventional coronary angiography, peripheral angiography and percutaneous intervention, and vascular medicine. His research interests are focused on peripheral artery disease. He has been involved in multiple clinical trials involving patients with atherosclerotic disease and has published several peer-reviewed articles and reviews. Dr. Gutierrez received his Bachelor of Arts from the College of the Holy Cross in 2000, Doctorate of Medicine from Case Western Reserve University School of Medicine in 2007, and Masters of Health Science in Clinical Research from Duke University School of Medicine in 2012. He completed his residency in Internal Medicine at Duke University Medical Center in 2010 and was appointed Chief Medical Resident of the Durham VA Medical Center in 2011. Dr. Gutierrez completed his General Cardiology and Vascular Medicine Fellowships at Brigham and Women’s Hospital and a Clinical Research Fellowship at the TIMI Study Group. Most recently Dr. Gutierrez matriculated his Interventional Cardiology Fellowship at Duke University Medical Center. Dr. Gutierrez is currently an Assistant Professor of Medicine at Duke University Hospital and Staff Physician at the Durham VA Medical Center. About STEMI Acute STEMI is a leading cause of cardiovascular death and remains a primary cause of the development of heart failure. Standard treatment of a STEMI involves PCI, during which a catheter is inserted into the artery to remove the blockage and restore blood flow. Following a STEMI, one of the critical factors influencing patient outcomes is reperfusion injury, which occurs when the oxygen-rich blood supply returns to the ischemic heart muscle. About FDY-5301 FDY-5301 is an elemental reducing agent containing sodium iodide for which Faraday has obtained method of use patent protection in major markets worldwide. Faraday has selected FDY-5301 for investigation in the belief that its properties are well-suited to mitigate ischemia-reperfusion injury (IRI). In preclinical IRI models, FDY-5301 reduced tissue damage, infarct size, and inflammation. FDY-5301 functions as a catalytic neutralizer of hydrogen peroxide, a prominent reactive oxygen species implicated in the IRI cascade leading to cardiomyocyte death, and also acts as an immunomodulating agent. A Phase 2 clinical trial of FDY-5301 in STEMI patients demonstrated that it was well-tolerated and provided encouraging signals of potential efficacy in minimizing cardiac damage. Results from that trial — known as Iocyte AMI — were reported in the January 15, 2022, issue of the International Journal of Cardiology. About Faraday Pharmaceuticals, Inc. Faraday Pharmaceuticals® is a clinical-stage biopharmaceutical company focused on the prevention of heart failure by reducing myocardial damage in acute STEMI. The company was founded by Dr. Mark Roth of the Fred Hutch Cancer Center and is backed by an investor group led by ARCH Venture Partners and Polaris Partners. The company’s lead program, FDY-5301, is in a pivotal Phase 3 trial and is designed to reduce IRI in acute STEMI, a leading cause of death and a primary cause of the development of heart failure. The company is headquartered in Seattle. For more information, visit www.faradaypharma.com or follow the company on LinkedIn. Contact:Brian BlackmanChief Financial Officerbblackman@faradaypharma.com Contact:PJ KelleherLifeSci Advisors, LLC+1-617-430-7579pkelleher@lifesciadvisors.com
Coronary/Structural Heart
SCCT 2024: HeartFlow to Present New Data on Coronary Artery Disease Management with Coronary Computed Tomography Angiography
MOUNTAIN VIEW, Calif., July 16, 2024 (GLOBE NEWSWIRE) — HeartFlow, a leader in non-invasive artificial intelligence (AI) heart care solutions, today announced it will be presenting new findings on the use of coronary computed tomography angiography (coronary CTA) in coronary artery disease (CAD) management and insights on CTA reimbursement at the upcoming 19th Annual Scientific Meeting of SCCT. The annual meeting will take place on July 18-21, 2024 in Washington, D.C. Subset data from the PRECISE (Prospective Randomized Trial of the Optimal Evaluation of Cardiac Symptoms and Revascularization) Trial will also be presented. The PRECISE clinical trial data, originally presented in late 2022, confirmed CTA + FFRCT increases diagnostic accuracy, reduces unnecessary testing and offers higher confidence in identifying patients needing treatment versus traditional testing (stress nuclear, stress echo, and invasive coronary angiography). The new subset data analyzes the association between body mass index, testing performance and clinical outcomes in patients with stable chest pain. New insights from the ADVANCE Registry, including analysis on the relationship between risk factors and quantified atherosclerotic burden and differences in plaque quantification in a Japanese patient population will be shared. Along with the significant work HeartFlow has undertaken to develop age and sex specific nomograms, these data will be a pivotal step to better stratify patients and impact treatment decisions going forward. “The critical role of CTA in the diagnosis and management of coronary artery disease continues to drive every decision we make, ensuring the right patients are getting the right care at the right time,” said Campbell Rogers, Chief Medical Officer of HeartFlow. “This year, we are excited to share the latest insights on the importance of quantifying coronary artery plaque, as well as changes in the reimbursement landscape. We are pleased to continue providing robust clinical evidence as to how our products can help health care providers manage their patient’s heart health.” Details of the presentations are as follows: Session: Poster Session 8 – CT in Non-Acute Chest PainTitle: “Association Between Body Mass Index, Testing Performance And Clinical Outcomes In Patients With Stable Chest Pain: Insights From The PRECISE Trial”Date: Friday, July 19, 2024Time: 9:30 – 10:15 AM EDTLocation: Exhibit Hall Session: Poster Session 16 – Plaque ImagingTitle: The Relationship Of Risk Factors And Quantified Atherosclerotic Burden On Coronary Computed Tomographic Angiography – Lessons From The ADVANCE RegistryDate: Saturday, July 20 Time: 9:30 – 10:35 AM EDTLocation: Exhibit Hall Session: Poster Session 16 – Plaque ImagingTitle: Quantitative Differences Between Japanese And Non-Japanese Patients’ Coronary Computed Tomographic Angiography Derived Plaque – Insights From The ADVANCE RegistryDate: Saturday, July 20 Time: 9:30 – 10:35 AM EDTLocation: Exhibit Hall Session: Business Aspects of Cardiac CT – Part TwoTitle: “How clinicians can work with administrators to maximize cardiac CT reimbursement” Presenter: Cara SantilloDate: Thursday, July 18, 2024Time: 4:05 PM – 4:20 PM EDT Location: Liberty N-P Heartflow invites attendees to two sponsored events, including “Women in CT Happy Hour,” which will take place on July 18 from 6:00 PM – 7:00 PM EDT in the 2nd Floor Lobby, and “The Power of HeartFlow AI in the Management of CAD from Diagnosis to Treatment” symposium, which will take place on July 19 from 11:55 AM – 12:45 PM EDT in the Capitol/Congress Room. About HeartFlow, Inc.HeartFlow is transforming precision coronary care with the only AI-powered non-invasive integrated heart care solution across the CCTA pathway. As the pioneer of FFRCT, which is now supported by the ACC/AHA Chest Pain Guideline, HeartFlow continues to advance the diagnosis and management of CAD. HeartFlow’s suite of non-invasive technologies includes its FFRCT Analysis, RoadMap™Analysis, and Plaque Analysis. To date, more than 500 peer-reviewed publications have validated our approach and, more importantly, our technologies have helped clinicians diagnose and manage over 250,000 patients. For more information, visit www.heartflow.com. Media ContactLinly KuSr. Digital Marketing Managermedia@heartflow.com Investor ContactNick Laudico VP of Business Development and Investor Relationsnlaudico@heartflow.com
Cytokinetics Announces Seven Upcoming Presentations at the European Society of Cardiology Congress 2024
Late Breaking Clinical Trial Presentations to Feature Additional Results from SEQUOIA-HCM Related to Patient-Reported Health Status, Cardiac Structure and Function and Biomarkers Analyses of Safety and Outcomes from FOREST-HCM Related to Withdrawal of Standard of Care Medications to be Presented SOUTH SAN FRANCISCO, Calif., July 16, 2024 (GLOBE NEWSWIRE) — Cytokinetics, Incorporated (Nasdaq: CYTK) today announced seven upcoming presentations, including four Late Breaking Clinical Trial presentations related to aficamten, at the European Society of Cardiology Congress 2024, taking place in London, UK from August 30, 2024 – September 2, 2024. Late Breaking Clinical Trials Title: Effect of Aficamten on Patient-Reported Health Status in Obstructive Hypertrophic Cardiomyopathy: Results from SEQUOIA-HCMPresenter: John A. Spertus, M.D., M.P.H., Professor, Daniel J. Lauer Missouri Endowed Chair in Metabolic and Vascular Disease Research, Clinical Director, University of Missouri Kansas City Healthcare Institute for Innovations in Quality and Saint Luke’s Mid America Heart InstituteDate: September 1, 2024Topic: European Society of Cardiology Session Title: Trial Updates and Other Studies in Hypertrophic Cardiomyopathy and Aortic StenosisSession Type: Late-Breaking ScienceSession Time: 8:15-9:45 AM BSTPresentation Time: 8:15 AM BSTLocation: Bishkek Title: Impact of Aficamten on Echocardiographic Cardiac Structure and Function in Adults with Symptomatic Obstructive Hypertrophic CardiomyopathyPresenter: Sheila Hegde, M.D., M.P.H., Cardiovascular Medicine Specialist, Division of Cardiovascular Medicine, Brigham and Women’s HospitalDate: September 1, 2024Topic: European Society of Cardiology Session Title: Trial Updates and Other Studies in Hypertrophic Cardiomyopathy and Aortic StenosisSession Type: Late-Breaking ScienceSession Time: 8:15-9:45 AM BSTPresentation Time: 8:26 AM BSTLocation: Bishkek Title: Effect of Aficamten on Structure and Function in Patients with Obstructive Hypertrophic Cardiomyopathy: The SEQUOIA-HCM CMR SubstudyPresenter: Ahmad Masri, M.D., MS, Director of the Hypertrophic Cardiomyopathy Center at Oregon Health & Science UniversityDate: September 1, 2024Topic: European Society of Cardiology Session Title: Trial Updates and Other Studies in Hypertrophic Cardiomyopathy and Aortic StenosisSession Type: Late-Breaking ScienceSession Time: 8:15-9:45 AM BSTPresentation Time: 8:37 AM BSTLocation: Bishkek Title: Safety and Outcomes of Standard of Care Medications Withdrawal in Patients with Obstructive Hypertrophic Cardiomyopathy Treated with Aficamten in FOREST-HCM TrialPresenter: Ahmad Masri, M.D., MS, Director of the Hypertrophic Cardiomyopathy Center at Oregon Health & Science UniversityDate: September 1, 2024Topic: European Society of Cardiology Session Title: Trial Updates and Other Studies in Hypertrophic Cardiomyopathy and Aortic StenosisSession Type: Late-Breaking ScienceSession Time: 8:15-9:45 AM BSTPresentation Time: 8:48 AM BSTLocation: Bishkek Oral Presentations Title: Clinical Application of Biomarkers in Obstructive Hypertrophic Cardiomyopathy: Insights from SEQUOIA-HCMPresenter: Caroline Coats, M.D., Ph.D., Lead Clinician, West of Scotland Inherited Cardiac Conditions Service, Honorary Senior Lecturer, School of Cardiovascular and Metabolic Health, University of GlasgowDate: September 1, 2024Topic: Hypertrophic Cardiomyopathy Session Title: Novel Therapies for Hypertrophic Cardiomyopathy: Recent Developments and Future ProspectsSession Type: Advances in ScienceSession Time: 8:15-9:45 AM BSTPresentation Time: 8:51 AM BSTLocation: Dublin Title: Aficamten in Patients with Obstructive Hypertrophic Cardiomyopathy: An Integrated Safety AnalysisPresenter: Ahmad Masri, M.D., MS, Director of the Hypertrophic Cardiomyopathy Center at Oregon Health & Science UniversityDate: September 1, 2024Topic: Hypertrophic Cardiomyopathy Session Title: Novel Therapies for Hypertrophic Cardiomyopathy: Recent Developments and Future ProspectsSession Type: Advances in ScienceSession Time: 8:15-9:45 AM BSTPresentation Time: 9:09 AM BSTLocation: Dublin Moderated ePoster Title: Menopausal Status and Clinical Outcomes in Women with Heart Failure with Reduced Ejection Fraction: the GALACTIC-HF TrialPresenter: Maria A. Pabon, M.D., Instructor of Medicine, Brigham and Women’s Hospital, Harvard Medical School; Associate Director, Cardiac Imaging Core LabDate: September 2, 2024Topic: Cardiovascular Disease in Women Session Title: Cardiovascular Disease in WomenSession Type: Moderated ePostersSession Time: 9:00-9:50 AM BSTLocation: Station 6 About Cytokinetics Cytokinetics is a late-stage, specialty cardiovascular biopharmaceutical company focused on discovering, developing and commercializing first-in-class muscle activators and next-in-class muscle inhibitors as potential treatments for debilitating diseases in which cardiac muscle performance is compromised. As a leader in muscle biology and the mechanics of muscle performance, the company is developing small molecule drug candidates specifically engineered to impact myocardial muscle function and contractility. Cytokinetics is preparing for regulatory submissions for aficamten, its next-in-class cardiac myosin inhibitor, following positive results from SEQUOIA-HCM, the pivotal Phase 3 clinical trial in obstructive hypertrophic cardiomyopathy. Aficamten is also currently being evaluated in MAPLE-HCM, a Phase 3 clinical trial of aficamten as monotherapy compared to metoprolol as monotherapy in patients with obstructive HCM, ACACIA-HCM, a Phase 3 clinical trial of aficamten in patients with non-obstructive HCM, CEDAR-HCM, a clinical trial of aficamten in a pediatric population with obstructive HCM, and FOREST-HCM, an open-label extension clinical study of aficamten in patients with HCM. Cytokinetics is also developing omecamtiv mecarbil, a cardiac muscle activator, in patients with heart failure. Additionally, Cytokinetics is developing CK-586, a cardiac myosin inhibitor with a mechanism of action distinct from aficamten for the potential treatment of HFpEF, and CK-136, a cardiac troponin activator for the potential treatment HFrEF and other types of heart failure, such as right ventricular failure resulting from impaired cardiac contractility. For additional information about Cytokinetics, visit www.cytokinetics.com and follow us on X, LinkedIn, Facebook and YouTube. Forward-Looking Statements This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the “Act”). Cytokinetics disclaims any intent or obligation to update these forward-looking statements and claims the protection of the Act’s Safe Harbor for forward-looking statements. Examples of such statements include, but are not limited to, statements relating to any of our other clinical trials, statements relating to the potential benefits of omecamtiv mecarbil, aficamten, or any of our other drug candidates. Cytokinetics’ research and development activities; the design, timing, results, significance and utility of preclinical and clinical results; and the properties and potential benefits of Cytokinetics’ other drug candidates. Such statements are based on management’s current expectations, but actual results may differ materially due to various risks and uncertainties, including, but not limited to, potential difficulties or delays in the development, testing, regulatory approvals for trial commencement, progression or product sale or manufacturing, or production of Cytokinetics’ drug candidates that could slow or prevent clinical development or product approval; Cytokinetics’ drug candidates may have adverse side effects or inadequate therapeutic efficacy; the FDA or foreign regulatory agencies may delay or limit Cytokinetics’ ability to conduct clinical trials; Cytokinetics may be unable to obtain or maintain patent or trade secret protection for its intellectual property; standards of care may change, rendering Cytokinetics’ drug candidates obsolete; and competitive products or alternative therapies may be developed by others for the treatment of indications Cytokinetics’ drug candidates and potential drug candidates may target. For further information regarding these and other risks related to Cytokinetics’ business, investors should consult Cytokinetics’ filings with the Securities and Exchange Commission. CYTOKINETICS® and the CYTOKINETICS and C-shaped logo are registered trademarks of Cytokinetics in the U.S. and certain other countries. Contact:Cytokinetics Diane WeiserSenior Vice President, Corporate Affairs(415) 290-7757
Sheba Startup Innovalve Bio Medical Ltd Acquired by Edwards Lifesciences Corporation
RAMAT GAN, Israel – July 15, 2024 – ARC Innovation at Sheba Medical Center, Israel’s largest medical center announced today another successful startup exit from within its innovation ecosystem with the acquisition of Innovalve Bio Medical Ltd by Edwards Lifesciences Corporation (NYSE: EW), a global leader in patient-focused medical innovations […]
Lexeo Therapeutics Announces Positive Interim Phase 1/2 Clinical Data of LX2006 for the Treatment of Friedreich Ataxia Cardiomyopathy
Achieved mean reduction in left ventricular mass index (LVMI) of 11.4% at 12 months and 18.3% at 18 months in participants with elevated LVMI at baseline >10% reduction in LVMI at 12 months in 75% of participants with elevated LVMI at baseline Sustained and consistent improvements in other key measures of cardiac status, including left ventricular wall thickness and troponin I, in majority of participants at 12 months Increased post-treatment frataxin expression above baseline in all participants evaluated via myocardial biopsy to date LX2006 was well tolerated with no treatment-related serious adverse events to date; proceeding to Cohort 3 in SUNRISE-FA, with one participant dosed in this cohort to date Company to host webcast today at 8:00 AM ET NEW YORK, July 15, 2024 (GLOBE NEWSWIRE) — Lexeo Therapeutics, Inc. (Nasdaq: LXEO), a clinical stage genetic medicine company dedicated to pioneering treatments for genetically defined cardiovascular diseases and APOE4-associated Alzheimer’s disease, today announced positive interim data of LX2006 for the treatment of Friedreich ataxia (FA) cardiomyopathy. Across both the Lexeo SUNRISE-FA Phase 1/2 clinical trial (NCT05445323) and the Weill Cornell Medicine investigator-initiated Phase 1A trial (NCT05302271), LX2006 was well tolerated with no treatment-related serious adverse events, and clinically meaningful improvements in cardiac biomarkers were observed with increasing improvement over time. “We are very encouraged by these data and the potential of LX2006 to treat FA cardiomyopathy, a devastating and fatal condition with no currently approved therapies,” said Dr. Eric Adler, Chief Medical Officer and Head of Research at Lexeo Therapeutics. “Based on the favorable safety profile and clinical benefits observed to date, we are excited to explore expedited clinical development of LX2006, including potential for accelerated approval of this possibly life-saving treatment.” “The interim data shared today demonstrate clinically meaningful improvements across multiple cardiac biomarkers of hypertrophy, a hallmark of FA cardiomyopathy,” said Dr. Sandi See Tai, Chief Development Officer at Lexeo. “Together with the increases in frataxin protein expression observed in SUNRISE-FA cardiac biopsies to date, these results further highlight the potential of LX2006 to positively impact outcomes for people with FA cardiomyopathy. I would like to thank the participants, caregivers, and investigators participating in these trials who have helped to achieve this important milestone.” FA cardiomyopathy is a devastating, rare, and progressive disorder caused by loss of function mutations in the frataxin gene. Thus far in participants in the SUNRISE-FA trial with cardiac biopsies, low levels of frataxin have been found in the heart at baseline, estimated to be 2% or less of normal. In terms of clinical presentation, FA cardiomyopathy is typically characterized by left ventricular hypertrophy ultimately progressing to heart failure, and cardiac dysfunction is the cause of death in up to 80% of individuals with FA. A new natural history subset analysis conducted by Lexeo showed elevated left ventricular mass index (LVMI) in adults with FA cardiomyopathy, and LVMI remained stable or increased with age without spontaneous improvement. Elevated LVMI is an indicator of left ventricular hypertrophy and correlated with mortality in multiple cardiovascular conditions including FA cardiomyopathy. Interim Safety Results LX2006 was well tolerated with no treatment-related serious adverse events to date in either studyNo signs of complement activation or other immunogenicity observedNo cardiac or hepatic safety signals observedAll adverse events were transient and resolvedNo participants discontinued from either study Interim Clinical Results (from 8 participants with > 6-months of follow-up) Left ventricular mass index (LVMI): Of participants with elevated LVMI at baseline, 75% achieved >10% reduction at 12 months (n=4). Of all participants, 50% achieved >10% reduction in LVMI at 12 months (n=6). Among the participants with elevated LVMI at baseline, mean reduction in LVMI was 11.4% at 12 months (n=4) and 18.3% at 18 months (n=2). Left ventricular (LV) lateral wall thickness: wall thickening, an early indicator of left ventricular hypertrophy, was reduced by 13.6% on average in all participants at 12 months (n=6).High-sensitivity Troponin I (hsTnI): troponin I, a biomarker of myocardial injury, was reduced by 53.3% on average in all participants at 12 months (n=5).Frataxin protein expression evaluated via myocardial biopsy: observed increased frataxin levels compared to baseline following treatment with LX2006 in all participants evaluated to date utilizing two distinct measurements: LCMS: frataxin increase observed in 3 of 3 evaluable participants.IHC: frataxin increase observed in 2 of 2 evaluable participants. Dosing Update and Next Steps As of July 15, 2024, 13 participants dosed to date across two trials: Cohort 1 (1.8x1011vg/kg): n=6Cohort 2 (5.6×1011 vg/kg): n=6Cohort 3 (1.2×1012 vg/kg): n=1 SUNRISE-FA independent Data and Safety Monitoring Board endorsed proceeding to Cohort 3 dose level (1.2x1012vg/kg). This cohort has started enrollment with 1 participant dosed to date and will include at least 3 participants.The Weill Cornell investigator-initiated trial is currently enrolling in Cohort 2.Lexeo expects to share further details of these interim results, including an additional cardiac biopsy from Cohort 2, at a scientific conference in Fall 2024. Corporate Webcast DetailsLexeo Therapeutics will host a webcast at 8:00 AM ET today, July 15, 2024. Analysts and investors can participate by accessing the webcast live here or on the News & Events page in the Investors section of Lexeo’s website, www.lexeotx.com. The webcast will be archived on the company’s website following the completion of the call. About the Clinical Studies SUNRISE-FA is a Lexeo-sponsored, multicenter, 52-week, open-label trial evaluating the safety and preliminary efficacy of LX2006 in people who have FA cardiomyopathy, with three ascending-dose cohorts. Investigators at Weill Cornell Medicine are conducting a Phase 1A study of AAVrh.10hFXN, known as LX2006 at Lexeo, in a single-site, 52-week, open-label trial evaluating the safety and preliminary efficacy in people who have FA cardiomyopathy, in two ascending-dose cohorts with five participants per cohort. About LX2006LX2006 is an AAV-based gene therapy candidate delivered intravenously for the treatment of FA cardiomyopathy, the most common cause of mortality in individuals with FA affecting approximately 5,000 people in the United States. LX2006 is designed to target the cardiac manifestations of FA by delivering a functional frataxin gene to promote the expression of the frataxin protein and restore mitochondrial function in myocardial cells. In preclinical studies, LX2006 reversed the cardiac abnormalities in FA disease models and showed improvement in cardiac function and survival while demonstrating a favorable safety profile. The FDA has granted Rare Pediatric Disease designation, Fast Track designation, and Orphan Drug designation to LX2006 for the treatment of FA cardiomyopathy. About Lexeo Therapeutics Lexeo Therapeutics is a New York City-based, clinical stage genetic medicine company dedicated to transforming healthcare by applying pioneering science to fundamentally change how genetically defined cardiovascular diseases and APOE4-associated Alzheimer’s disease are treated. Using a stepwise development approach, Lexeo is leveraging early proof-of-concept functional and biomarker data to advance a pipeline of cardiovascular and APOE4-associated Alzheimer’s disease programs. Cautionary Note Regarding Forward-Looking StatementsCertain statements in this press release may constitute “forward-looking statements” within the meaning of the federal securities laws, including, but not limited to, our expectations and plans regarding our current product candidates and programs, including statements regarding the potential benefits of LX2006 for the treatment of Friedreich ataxia cardiomyopathy and the timing for receipt and announcement of data from its clinical trials, and the timing and likelihood of potential regulatory approval. Words such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “develop,” “plan” or the negative of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While Lexeo believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements. These forward-looking statements are based upon current information available to the company as well as certain estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Lexeo’s filings with the U.S. Securities and Exchange Commission (SEC)), many of which are beyond the company’s control and subject to change. Actual results could be materially different from those indicated by such forward-looking statements as a result of many factors, including but not limited to: risks and uncertainties related to global macroeconomic conditions and related volatility; expectations regarding the initiation, progress, and expected results of Lexeo’s preclinical studies, clinical trials and research and development programs; the unpredictable relationship between preclinical study results and clinical study results; delays in submission of regulatory filings or failure to receive regulatory approval; liquidity and capital resources; and other risks and uncertainties identified in Lexeo’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2023, filed with the SEC on May 9, 2024, and subsequent future filings Lexeo may make with the SEC. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Lexeo claims the protection of the Safe Harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements. Lexeo expressly disclaims any obligation to update or alter any statements whether as a result of new information, future events or otherwise, except as required by law. Media Response:Janine Bogris (201) 245-6838 janine.bogris@inizioevoke.com Investor Response:Stephen Jasper(858) 525-2047stephen@gilmartinir.com
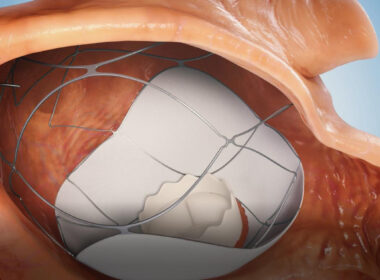
A New Era in Heart Surgery – Israeli Biomed Company TruLeaf Medical’s Two Needle Sticks Procedure for Mitral Valve Replacement
AllMeD Solutions (TASE:ALMD) subsidiary TruLeaf Medical receives Helsinki Ethics Committee’s approval in Uzbekistan to conduct clinical trial in human subjects, paving the way for minimally invasive heart valve treatmentsCAESAREA, Israel, July 11, 2024 /PRNewswire/ — AllMeD Solutions announced subsidiary TruLeaf Medical’s receipt of the Helsinki Ethics Committee’s approval in Uzbekistan to conduct clinical trial in human subjects. As part of the trial, a prosthetic mitral valve will be implanted via two needle sticks in the groins in a two-stage catheterization procedure without the need for open-heart surgery (transcatheter mitral valve replacement, TMVR). The implantation of the innovative platform (the RoseDoc) developed by TruLeaf, which replaces the patient’s leaky heart valve, will be carried out in two stages. In the first stage, a docking station will be implanted in the left atrium, followed a few weeks later by implantation of an artificial ‘biological’ mitral valve prosthesis.
Continue Reading
Trueleaf’s Medical Device
Today, there are tens of millions of patients with severe, life-threatening mitral valve regurgitation (leaky valve) across the world. This leak causes heart failure, heart arrhythmia, brain strokes leading to high mortality. About 10% of the world’s population over the age of 75 suffer from a leaky mitral heart valve. In the U.S. alone there are about 4 million patients.
These patients experience a substantial decrease in their functional capacity manifesting, such as fatigue, shortness of breath on exertion with lower and lower exertion and arrhythmias, progressively impairing their daily routine. Today, the most effective treatment for these patients is complex open-heart surgery to repair or replace the leaky heart valve. However, it is only offered to about 2% of patients due to the high surgical risk.
The unique RoseDoc platform developed by TruLeaf is the first-of-its-kind technology allowing implantation of a biological bioprosthesis to replace the diseased valve through catheterization only. This ground-breaking procedure is minimally invasive, performed on a beating heart via two needle punctures without surgery or the use of a heart-lung machine. As such, it is associated with substantially lower risk compared to the traditional open-heart mitral valve surgery. As a result, millions of patients around the world, who until now were deemed inoperable, will be able to get receive a new valve and experience a significant improvement in their functional capacity, quality of life and life expectancy, allowing them to resume normal life.As part of the preparations for human implantations in clinical trials in Uzbekistan, TruLeaf conducted additional implantations in large animals with the participation of Dr. Horst Sievert, one of the world’s leading interventional cardiologists, who is expected to lead TruLeaf’s clinical trials.TruLeaf Medical, Ltd was founded in 2017 by three Israeli entrepreneurs – Benjamin Spencer, Nathaniel Benisho and the late Dr. Uri Rosenstein. Benjamin and Nathaniel’s played a seminal role in the development of the first ever transcatheter aortic bioprosthesis, the Sapien valve, initially within the Israeli company PVT, that was later acquired by the medical technology giant Edwards Lifesciences. Today, the aortic valve that Benjamin and Nathaniel developed saves thousands of lives of patients with aortic stenosis every year all over the world.Benjamin Spencer, TruLeaf Medical CEO, explains, “The main challenge with existing transcatheter TMVR technologies is achieving optimal anchoring of the valve prosthesis to the heart, given the complex anatomy and physiology of the native mitral valve. The RoseDoc TMVR platform is technically simple, safe, and has proven effective in long-term animal testing. Completely eliminating the leak prevents the progressive dilation of the heart, which otherwise worsens the leak in a vicious cycle, leading to further weakening of the heart muscle and intractable heart failure. Currently, patients with severe mitral valve leaks that are unresponsive to maximal medical treatment have no effective options. The vast majority of these patients are declined surgery due to prohibitive risk. The unique RoseDoc TMVR platform provides a potential lifeline for these patients.”Professor Oz Shapira, AllMeD Solutions CEO, adds, “As a heart surgeon who has performed hundreds of open-heart surgeries to treat leaky heart valves, the possibility of replacing the mitral valve through a simple and quick needle puncture operation is a true revolution that may offer a solution to millions who currently have no other option.””The first-in-human trial is both exciting and mission-critical for TruLeaf’s success. Given the outstanding results of the preclinical experiments, I am confident that TruLeaf’s innovative RoseDoc TMVR platform will perform exceptionally well in humans and eventually save countless lives of patients who currently have no alternatives. AllMeD Solutions will continue to demonstrate its ability to identify early-stage startups and leverage its vast knowledge, experience, and expertise in the med-tech space to lead these companies to engineering, clinical, and business success.”Photo: https://mma.prnewswire.com/media/2459133/AllMeD_Solutions.jpgLogo: https://mma.prnewswire.com/media/2459132/AllMeD_Solutions_Logo.jpgSOURCE AllMeD Solutions
XyloCor Therapeutics and SmartCella Enter into License Agreement for Use of the Extroducer Infusion Catheter System to Administer Novel Gene Therapy XC001 to the Heart
– The Extroducer® Infusion Catheter System ® enables local delivery of XC001 to the heart without the need for surgery. – XC001 has achieved positive Phase 1/2 results in the EXACT Trial validating its transformative potential for treatment of refractory angina in patients who have exhausted available treatment options and […]
CARMAT accelerates its sales momentum and reiterates its confidence in its development outlook
20 implants of the Aeson® artificial heart performed in the first half of 2024 Pace of 4 implants per month in the second quarter Half-year sales at €3.2 million, higher than the 2023 full-year sales Unique safety and performance profile of Aeson® confirmed, based on more than 70 implants made since inception […]
Pulnovo Medical Announces Joining PHA’s (Pulmonary Hypertension Association) Corporate Committee
SHANGHAI, July 9, 2024 /PRNewswire/ — Pulnovo Medical, a globally recognized device pioneer in the treatment for pulmonary hypertension and heart failure, has recently announced its partnership with the Pulmonary Hypertension Association (PHA). This partnership not only underscores Pulnovo Medical’s growing global influence but also empowers PHA’s mission to extend and improve the lives of those affected by pulmonary hypertension (PH) worldwide.
Pulnovo Medical and the PHA are united by a collective vision to a future where pulmonary hypertension is better understood and more efficiently treated. Pulnovo Medical’s PADN technology, an innovative interventional therapy in the field of pulmonary hypertension, provides a new and beneficial solution for patients troubled by traditional drug treatments. Currently Pulnovo Medical is conducting global trials in US and Europe. This strategic partnership aims to combine Pulnovo Medical’s cutting-edge PADN therapies for Pulmonary Hypertension with PHA’s extensive network to enhance public awareness and improve the care of PH patients globally. As a member of PHA, Pulnovo Medical will actively participate in PHA’s educational programs, research projects and advocacy campaigns. Our collaboration will harness the urgency of our shared mission to make a lasting impact on pulmonary hypertension patients worldwide.
About PHA
The PHA was founded in 1991 in Florida and evolved into an international community of more than 16,000 people with PH, caregivers, family members and health care professionals. As one of the largest and oldest pulmonary hypertension association in the world, the PHA acts as a trusted liaison between the pulmonary hypertension community and industry and academic partners.
About PADN
Pulmonary Artery Denervation (PADN) is a percutaneous pulmonary artery intervention procedure that uses a PADN catheter to deliver radiofrequency energy to the sympathetic nerves in the outer membrane of the pulmonary artery, resulting in the disappearance of the myelin sheath of the nerves and the fusion of the axons, which inhibits sympathetic activity, increases cardiac output, reduces pulmonary artery pressure, inhibits the pathologic remodeling of the pulmonary arteries, improves the patient’s exercise endurance and cardiac function, and achieves long-term benefits from a single minimally invasive procedure.
About Pulnovo Medical
Pulnovo Medical Limited, is a globally recognized device pioneer in the treatment for pulmonary hypertension and heart failure, is committed to leveraging our deep expertise in the science of breakthrough technologies with the goal to market our innovative therapeutic solutions and benefit patients around the world.
For more information, please visit: www.pulnovomed.us
Reflow Medical Enrolls First Patients in Pilot Study of Coronary Sirolimus-Eluting Retrievable Scaffold System (Spur Elute)
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical, Inc., a developer of innovative devices focused on cardiovascular disease, announces the first patient enrollments in “A pilot study of the Drug-Eluting Coronary Spur™ StEnt as a Primary trEatment for in-stent Restenosis (ISR) of the CORONARY arteries” (DEEPER CORONARY, NCT06117150). ISR is a common clinical problem that can generate significant […]