LONDON–(BUSINESS WIRE)–Silence Therapeutics plc, Nasdaq: SLN (“Silence” or the “Company”), an experienced and innovative biotechnology company committed to transforming people’s lives by silencing diseases through precision engineered medicines, today announced positive topline 48-week data from the ALPACAR-360 phase 2 study of zerlasiran (SLN360) in 178 subjects with baseline lipoprotein(a), or […]
Coronary/Structural Heart
Haemonetics Launches Limited Market Release for New VASCADE MVP XL Vascular Closure Device
BOSTON, June 18, 2024 /PRNewswire/ — Haemonetics Corporation (NYSE: HAE), a global medical technology company focused on delivering innovative solutions to drive better patient outcomes, has launched a limited market release of its new VASCADE MVP® XL mid-bore venous closure device. The VASCADE MVP XL system expands Haemonetics’ VASCADE® portfolio of vascular closure systems featuring an innovative collapsible disc technology and a proprietary resorbable collagen patch designed to promote rapid hemostasis.
Haemonetics’ current VASCADE portfolio includes the VASCADE system, designed for “small-bore” femoral arterial and venous closure with standard 5-6/7F procedural sheaths, and the VASCADE MVP® system, designed for “mid-bore” multi-access femoral venous closure with 6-12F procedural sheaths. The upsized VASCADE MVP XL system utilizes 58% more collagen and a larger disc than the current VASCADE MVP system, providing a robust closure solution for procedures requiring 10-12F sheaths (up to 15F in outer diameter) such as cryoablation and left atrial appendage closure for atrial fibrillation patients.
“With VASCADE MVP XL, Haemonetics continues to expand its presence and broaden its reach in the $2.7 billion total addressable market for vascular closure solutions,” said Stew Strong, President of Global Hospital at Haemonetics. “The introduction of VASCADE MVP XL underscores our commitment to innovation and improving patient care, as we enhance our range of vascular closure solutions to address increasing demand for catheter-based ablation technologies. We are enthusiastic about the initial launch of VASCADE MVP XL and anticipate a full market release later this year.”
The VASCADE MVP XL system earned pre-market approval from the U.S. Food and Drug Administration this spring. The limited market release follows the first procedure performed using VASCADE MVP XL by Dr. Tom McElderry, Section Chief, Electrophysiology and Co-Director Heart & Vascular Center at the University of Alabama at Birmingham.
About Haemonetics
Haemonetics (NYSE: HAE) is a global healthcare company dedicated to providing a suite of innovative medical products and solutions for customers, to help them improve patient care and reduce the cost of healthcare. Our technology addresses important medical markets: blood and plasma component collection, the surgical suite and hospital transfusion services. Haemonetics’ Global Hospital business provides a range of solutions to address the needs of hospitals, including Interventional Technologies for electrophysiology and interventional cardiology, and Blood Management Technologies that include diagnostics to help inform treatment decisions, technologies to help avoid unnecessary allogeneic transfusions and solutions to help optimize management of blood products. To learn more about Haemonetics, visit www.haemonetics.com.
Cautionary Statement Regarding Forward-Looking Information
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements. Forward-looking statements in this press release may include, without limitation, statements regarding plans and objectives of management for the operation of Haemonetics, including statements regarding potential benefits associated with the Vascade MVP XL vascular closure device and Haemonetics’ plans or objectives related to the commercialization of such product. Such forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon Haemonetics’ current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Factors that may influence or contribute to the inaccuracy of the forward-looking statements or cause actual results to differ materially from expected or desired results may include, without limitation, product quality; market acceptance; the effect of global economic and political conditions; and the impact of competitive products and pricing. These and other factors are identified and described in more detail in Haemonetics’ periodic reports and other filings with the U.S. Securities and Exchange Commission. Haemonetics does not undertake to update these forward-looking statements.
Investor Contacts:
Olga Guyette, Vice President-Investor Relations & Treasury
David Trenk, Manager-Investor Relations
(781) 356-9763
(203) 733-4987
[email protected]
[email protected]
Media Contact:
Josh Gitelson, Senior Director-Global Communications
(781) 356-9776
[email protected]
SOURCE Haemonetics Corporation
Morton Plant Hospital First in Tampa Bay to Use New Treatment for Restenosis
CLEARWATER, Fla., June 18, 2024 /PRNewswire/ — Morton Plant Hospital recently became the first hospital in the Tampa Bay area to use a drug-coated balloon to treat in-stent restenosis. The hospital was ranked #1 in Florida for Cardiac Surgery in 2023 by Healthgrades, where the area’s first robotic-assisted coronary artery bypass surgery took place in early June. BayCare Medical Group’s Interventional Cardiologist Lang Lin, MD, and her expert team of medical professionals at Morton Plant Hospital performed the first procedure with the drug-coated balloon earlier this month.
Continue Reading
Cardiologist Lang Lin, MD, and her team from Morton Plant Hospital’s cardiac catheterization lab celebrate using a new tool to treat restenosis in Clearwater, Fla.
Restenosis occurs when an artery that has previously been stented narrows again because of plaque or scar tissue.
Treating in-stent restenosis remains a significant challenge for both patients and cardiologists, even 20 years after drug-eluting stents (which are coated with medicine to keep arteries open) became the standard of care.” Dr. Lin said. Until now, in-stent restenosis has most commonly been treated in one of two ways: by placing an additional stent in the stent of the artery that has narrowed again or by performing coronary artery bypass surgery. The challenge with two or more layers of stents is that additional stents become less effective and more difficult to treat. Coronary artery bypass surgery has increased risks with older patients and sometimes limited bypass target vessels. The new drug-coated balloon, AGENT™ from Boston Scientific, was approved by the U.S. Food and Drug Administration (FDA) for commercial use in March. The balloon catheter, the first drug-coated coronary balloon in the United States, reopens the narrowed stent and releases the high concentration of drugs to prevent scar tissue from forming and blocking the artery wall again. “We are pleased to have the opportunity to offer this cutting-edge treatment to our patients,” Dr. Lin said. “The new technology will help us to treat certain patients with in-stent restenosis without the need for another stent, invasive surgery or brachytherapy (radiation).” For more information on heart and vascular services at BayCare: BayCare’s Heart and Vascular Services. About Morton Plant HospitalSince 1916, Morton Plant Hospital has been committed to improving the health of all it serves through community-owned health care services that set the standard for high-quality, compassionate care. The 599-bed hospital is proud to offer nationally recognized care delivered in more than 50 specialty areas. Part of BayCare Health System, Morton Plant Hospital offers innovative, accessible and quality services to provide our community with a lifetime of compassionate, convenient care. The hospital is located at 300 Pinellas Street in Clearwater, Florida. For more information, visit BayCare.org/MPH.About BayCareBayCare is a leading not-for-profit health care system that connects individuals and families to a wide range of services at 16 hospitals and hundreds of other convenient locations throughout the Tampa Bay and central Florida regions. The system is West Central Florida’s largest provider of behavioral health and pediatric services and its provider group, BayCare Medical Group, is one of the largest in the region. BayCare’s diverse network of ambulatory services includes laboratories, imaging, surgical centers, BayCare Urgent Care locations, wellness centers and one of Florida’s largest home care agencies, BayCare HomeCare. BayCare’s mission is to improve the health of all it serves through community-owned, health care services that set the standard for high-quality, compassionate care. For more information visit BayCare.org.SOURCE BayCare Health System
Promising long and short-term clinical data show potential of first ASD occluder with a bioresorbable, metal-free frame
atHeart Medical’s reSept ASD Occluder is designed to preserve patients’ future transseptal treatment optionsBAAR, Switzerland and SANTA CLARA, Calif., June 18, 2024 /PRNewswire/ — atHeart Medical, a medical device company establishing a new standard of care for atrial septal defects (ASDs) closure, today announced promising long and short-term clinical outcomes of its reSept ASD occluder, to be presented at the medical conference CSI Frankfurt. The data from three patient cohorts show positive efficacy and safety profile that contribute to a growing body of evidence about the innovative device’s potential. reSept is the first ASD occluder with a bioresorbable, metal-free frame, designed to enable future transseptal treatment: a true evolution for patients that need transcatheter septal closure.
Continue Reading
The reSept ASD Occluder has a bioresorbable, metal-free frame
A growing body of evidenceThe new reSept data to feature at CSI on June 19th include:
Positive ten-year outcomes from the first-in-human (FIH) trial showing long-term efficacy and safety of the device – to be presented by Dr. Kolja Sievert, from the CardioVascular Center Frankfurt (DE)
Promising two-year data showing complete closure in a case study from the University of Virginia (UVA) cohort of ASCENT ASD, the ongoing pivotal trial approved in the USA, Canada, France and Switzerland – discussed by Dr. Scott Lim, Professor of Medicine and Pediatrics at UVA
One-month reSept efficacy in patients with iatrogenic ASDs (iASDs), caused by mitral valve intervention, from the Canadian first-ever experience through Health Canada’s Medical Devices Special Access Program – shown by Dr. Fady Zaky from St Paul’s Hospital, Vancouver, (CA)
“Having pioneered reSept in its early days as part of ASCENT ASD in the USA, I am very pleased about the long-term outcomes from the earlier European FIH trial and the wealth of promising new data available,” said Dr. Saibal Kar, National Physician Director HCA Healthcare, USA, co-Principal Investigator of the ASCENT ASD trial. “They indicate how transcatheter ASD closure with a bioresorbable device could become an even less invasive procedure for patients long-term. This is a very exciting development for our patients.”To date around 100 patients with ASDs have been successfully treated as part of trials and special access iASD adult use in Canada, France, Germany, Switzerland and the USA. The ASCENT ASD pivotal study is currently enrolling patients in over 20 hospitals in the United States, four hospitals in France and will start treating patients in Canada and in Switzerland following Health Canada and Swissmedic’s recent authorizations.”Around 100 patients have been implanted with reSept to date. While hospitals continue to enroll patients as part of ASCENT ASD in several countries, we are excited that these positive outcomes showcase the potential for reSept to soon be the new standard of care,” said Laurent Grandidier, atHeart Medical CEO.The reSept ASD Occluder is an investigational device that aims to address the limitations of current occluders, which have metallic frames that stay in a patient’s heart for life and can limit transseptal interventions.About reSept™ ASD OccluderreSept is the first ASD occluder with a bioresorbable, metal-free frame, designed to enable future transseptal interventions. Unlike existing occluders, its frame comprises two synthetic fabric patches connected by bioresorbable filaments that overtime resorb in the body.About reSept clinical research programTo date, reSept has been implanted in around 100 patients as part of the following investigations:
First-in-Human trial in Germany – nine adult ASD patients
European registry in Switzerland and Germany – six ASD patients, of which four children
ASCENT ASD Pivotal trial – a prospective, single-arm, global multi-site clinical investigation approved by US FDA, French ANSM, Health Canada and Swissmedic; currently enrolling up to 250 patients, around 90 patients implanted to date. For more information: www.clinicaltrials.gov – Identifier: NCT04591392.
About atHeart MedicalatHeart Medical is a medical device company with offices in Switzerland and the United States committed to establish a new standard of care for treatment of atrial septal defects (ASDs). For more information: www.atheartmedical.com.About ASDCommonly described as a “hole in the heart”, an ASD is an opening in the heart septum between the left and right atria. ASDs are the second most common congenital heart defect, affecting six in 10,000 births.1 They can also be the result of procedures, such as mitral valve treatment, that require crossing the septum (iASDs). A large atrial septal defect can cause extra blood to overfill the lungs and overwork the right side of the heart. If not treated, it can lead to pulmonary hypertension, arrythmia, heart failure and increased risk of stroke.1 When ASDs require closure, the current standard of care is to implant a septal occluder with a metallic frame through a minimally invasive transcatheter procedure.SOURCE atHeart Medical
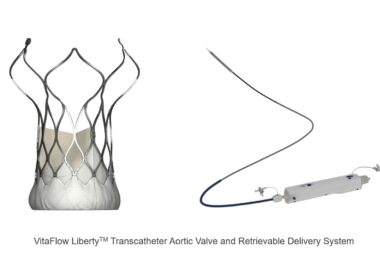
CardioFlow’s VitaFlow Liberty™ Granted EU CE-MDR Mark, Advancing Global Expansion Roadmap
SHANGHAI, June 17, 2024 /PRNewswire/ — MicroPort® CardioFlow Medtech Corporation (CardioFlow) (Stock Code: 02160.HK) recently announced that its self-developed second-generation transcatheter aortic valve implantation (TAVI) device, the VitaFlow LibertyTM Transcatheter Aortic Valve and Retrievable Delivery System (VitaFlow LibertyTM), has received EU CE-MDR certification. This certification highlights VitaFlow LibertyTM as a pioneering TAVI solution, that sets a new benchmark in transcatheter heart valve treatments.
Continue Reading
VitaFlow_Liberty__Transcatheter_Aortic_Valve_and_Retrievable_Delivery_System
With over 47 million patients globally[1] suffering from aortic valve stenosis and regurgitation, the prevalence rates of these conditions are on the rise due to an aging population. The TAVI solution provided by CardioFlow, which avoids open-heart surgery and offers various benefits like minimal trauma, quick recovery, and enhanced quality of life, is increasingly becoming a preferred choice for patients with aortic heart valve disease.
CardioFlow, one of the world’s leading innovative medical device companies, has entered the field of structural heart disease when the field was still at an early adoption phase. Originating and headquartered in Shanghai, China, CardioFlow was listed on the Hong Kong Stock Exchange on February 2021. The company has a diverse product pipeline resulting from independent and collaborative research, covering structural heart devices such as transcatheter aortic, mitral, and tricuspid valves, left atrial appendage occludes, and accessories. Leveraging its technological expertise and capacity for innovation, the company has successfully obtained approvals and launched several TAVI products globally, among which VitaFlow LibertyTM stands out as the world’s only electric retrievable transcatheter aortic valve system. The VitaFlowTM series TAVI solution along with its accessory – the AlwideTM series Balloon Catheter, has successfully covered nearly 700 core hospitals in 10 countries and regions, treating more than 10,000 patients with aortic valve disease worldwide.
The clinical data from VitaFlowTM series valves were revealed at PCR London Valves 2023, a leading global conference on structural heart diseases. These results highlight VitaFlowTM’s exceptional long-term clinical performance aligning with international top-tier standards. The long-term results of VitaFlowTM in high surgical risk patients with severe aortic stenosis showed promising outcomes in all-cause mortality, cardiac mortality, and permanent pacemaker implantation rates for patients over seven years, compared to other similar studies. During the conference, Dr. Darren Mylotte from Galway University Hospitals, Ireland, commented on the excellent data, and introduced the advantages of VitaFlow LibertyTM in its one of a kind motorized delivery system. The system can assist the valve to position easily due to its flexibility and 360° range of motion when treating complex anatomical patients with severe angled aortic arch deformities. The valve can also be fully retrieved and repositioned when released to 75%, and provides up to 3 retrievable opportunities for each procedure, thereby further optimizing the implantation effect. Additionally, it can effectively ensure the stability of valve release, reduce valve displacement, and make the procedure more controllable.Before launching into the EU market, VitaFlow LibertyTM conducted pre-market clinical implantations at Galway University Hospital in Ireland, Rigshospitalet (Copenhagen University Hospital) in Denmark, and St Thomas’ Hospital as well as Brighton & Sussex University Hospitals NHS Trust in the United Kingdom, and received very high appraisal from many well-known clinical professionals. Dr. Ole De Backer, a professor of interventional cardiology, who led the TAVI procedures at Rigshospitalet stated, “The overall release process of VitaFlow LibertyTM is notably stable, ensuring precise positioning. This stability is especially crucial in patients with a small left ventricles, where VitaFlow LibertyTM consistently achieves stable and precise deployment, fully demonstrating its distinct advantages. We look forward to its positive impact on a broader patient population following CE certification.” It has been reported that the European post-market clinical project will also be planned to start this year.As part of CardioFlow’s global expansion roadmap, the company has also achieved significant milestones with CE application on three of its products, including the AlwideTM Plus Balloon Catheter, an essential accessory for aortic valve procedures, as well as the AnchorManTM Left Atrial Appendage Closure System and the AnchorManTM Left Atrial Appendage Access System, both developed by its subsidiary, CardioAdvent.Jeff Lindstrom, President of CardioFlow, stated, “The certification of VitaFlow LibertyTM by the CE regulatory body under MDR, is a testament to CardioFlow’s world-class R&D, quality, and clinical capabilities. This recognition will expedite the global clinical adoption of the VitaFlowTM series along with other innovative products, advancing CardioFlow’s globalization strategy. This achievement also positions us to make a more substantial contributions to developments in the field of heart valve interventions, ultimately benefiting patients across the globe.”Guoming Chen, Chairman of CardioFlow, commented, “Securing the EU CE-MDR marking for VitaFlow LibertyTM is not just a passport for the product’s entry into the European market, it also represents a significant milestone in CardioFlow’s history and global roadmap. This achievement will assist in diversifying the company’s sources of sales revenue and bolstering our overall competitiveness with a steadfast commitment to world-class product innovation.”
1. Frost & Sullivan’s statistics, 2021
Photo – https://mma.prnewswire.com/media/2440350/VitaFlow_Liberty__Transcatheter_Aortic_Valve_and_Retrievable_Delivery_System.jpg
Aerovate Therapeutics Announces 24-Week Topline Results from the Phase 2b Portion of IMPAHCT Evaluating AV-101 for the Treatment of Pulmonary Arterial Hypertension
WALTHAM, Mass., June 17, 2024 (GLOBE NEWSWIRE) — Aerovate Therapeutics, Inc. (Nasdaq: AVTE) today announced topline results from the Phase 2b portion of the Inhaled iMatinib Pulmonary Arterial Hypertension Clinical Trial (IMPAHCT), a Phase 2b/Phase 3, randomized, double-blind, placebo-controlled, multi-national trial of AV-101, a novel dry powder inhaled formulation of imatinib, in adults with pulmonary arterial hypertension (PAH). The objective of the Phase 2b portion of IMPAHCT was to assess the efficacy, safety and tolerability of three different doses of AV-101 compared to placebo. The primary endpoint for the Phase 2b portion of IMPAHCT is change in PVR compared with placebo. Results showed that, while AV-101 was well tolerated across all dose groups, the study did not meet its primary endpoint for improvement in PVR compared to placebo for any of the studied doses or show meaningful improvements in the secondary endpoint of change in six minute walk distance (6MWD). Primary Endpoint – ITT analysis of PVR (dynes*sec/cm^5) DoseLeast-squares mean difference as compared with placebo (95% CI)P value10mg BID (N=50)42.8 (-80.57 to 166.09)0.496835mg BID (N=49)-5.5 (-129.16 to 118.18)0.930670mg BID (N=51)-57.0 (-181.14 to 67.20)0.3685 Secondary Endpoint – ITT analysis of 6MWD (meters) DoseLeast-squares mean difference as compared with placebo (95% CI)10mg BID (N=50)-11.7 (-34.75 to 11.26)35mg BID (N=49)-4.2 (-27.74 to 19.37)70mg BID (N=51)+1.3 (-22.09 to 24.60) The Company has also reviewed data from several additional secondary endpoints of the Phase 2b portion of IMPAHCT, which also failed to show meaningful improvements. Based upon these results, Aerovate, in agreement with the independent study advisory committee, is halting enrollment and shutting down the Phase 3 portion of IMPAHCT as well as the long-term extension study. “The results of the Phase 2b portion of IMPAHCT were unexpected and disappointing. Our immediate focus is on transparently sharing these findings with investigators, patients and the PAH community. In the coming weeks, we will engage closely with the IMPAHCT study advisory committee and the PAH community to thoroughly discuss these data and their implications,” said Tim Noyes, Chief Executive Officer of Aerovate. “We extend our heartfelt gratitude to all trial participants, investigators, and site teams for their dedication to advancing therapeutic options for the treatment of pulmonary arterial hypertension.” Aerovate plans to release full data from the Phase 2b portion of IMPAHCT at a later date, the timing of which is to be determined. As of June 15, 2024, Aerovate has approximately $100 million of cash, cash equivalents and short-term investments. About AV-101AV-101 is an investigational, proprietary dry powder inhaled formulation of the antiproliferative drug imatinib. Developed specifically for pulmonary arterial hypertension (PAH), AV-101 targets cellular hyperproliferation and resistance to apoptosis, driven by improper signaling in cells of the distal pulmonary arteries. AV-101 is designed for delivery by an easy-to-use dry powder inhaler, directly into the lungs to maximize potential clinical benefit and limit systemic adverse effects. About the IMPAHCT TrialIMPAHCT (Inhaled iMatinib Pulmonary Arterial Hypertension Clinical Trial) is a multi-national, placebo-controlled Phase 2b/Phase 3 trial in adults with PAH that continuously enrolled patients from Phase 2b to Phase 3. The Phase 2b portion of the trial evaluated three doses of AV-101 over 24 weeks, compared to placebo, to identify an optimal dose based on the primary endpoint, change in pulmonary vascular resistance (PVR), and safety, tolerability, and other clinical measures. More information about this trial is available at https://clinicaltrials.gov/ct2/show/NCT05036135. About Aerovate Therapeutics, Inc.Aerovate is a clinical stage biopharmaceutical company focused on developing drugs that meaningfully improve the lives of patients with rare cardiopulmonary disease. Aerovate’s initial focus is on advancing AV-101, its proprietary dry powder inhaled formulation of the drug imatinib for the treatment of patients with PAH. Learn more at aerovatetx.com or follow the Company on X (formerly known as Twitter) and LinkedIn. Available InformationAerovate announces material information to the public about the Company, its products and services, and other matters through a variety of means, including filings with the U.S. Securities and Exchange Commission (SEC), press releases, public conference calls, webcasts, the investor relations section of the Company website at ir.aerovatetx.com, and the Company’s X (formerly known as Twitter) account @AerovateTx in order to achieve broad, non-exclusionary distribution of information to the public and for complying with its disclosure obligations under Regulation FD. Cautionary Note Regarding Forward-Looking StatementsThis press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements can be identified by words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “future,” “goal,” “intend,” “look forward to,” “may,” “plan,” “potential,” “predict,” “project,” seek,” “strategy,” “should,” “target,” “will,” “would” and similar expressions regarding future periods. These forward-looking statements include, but are not limited to, statements regarding the Phase 2b/Phase 3 IMPAHCT, including the future release of full clinical data and the Company’s plan to halt the Phase 3 portion of the IMPAHCT trial. Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, those risks and uncertainties related to the therapeutic potential and clinical benefits of AV-101; the timing associated with the identification and activation of clinical sites, patient enrollment, initiation, delivery of drug supply and continuation of our Phase 2b/Phase 3 trial of AV-101 in PAH patients; the impact of public health crises on our business, clinical trials, operations and goals; positive results from a clinical study may not necessarily be predictive of the results of future or ongoing clinical studies; regulatory developments in the United States and foreign countries; as well as those risks and uncertainties set forth more fully under the caption “Risk Factors” in our most recent Annual Report on Form 10-Q filed with the SEC and subsequent filings with the SEC. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Any forward-looking statements contained in this press release represent our views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Media ContactMarites Coultermarites.coulter@vergescientific.com Investor ContactIR@Aerovatetx.com
Cytokinetics Announces Initiation of Phase 1 Study of Aficamten in Healthy Japanese Participants
SOUTH SAN FRANCISCO, Calif., June 17, 2024 (GLOBE NEWSWIRE) — Cytokinetics, Incorporated (Nasdaq: CYTK) today announced that the first participants have been dosed in a Phase 1 study evaluating the pharmacokinetics, safety and tolerability of aficamten in healthy Japanese and Caucasian participants. “We are conducting this Phase 1 bridging study to characterize the pharmacokinetics of aficamten in healthy Japanese adults and to gather evidence that we believe will be required for potential approval in Japan,” said Fady I. Malik, M.D., Ph.D., Cytokinetics’ Executive Vice President of Research & Development. “In parallel, we are continuing to execute on our later-stage global clinical development program for aficamten alongside preparing regulatory submissions in the U.S. and Europe which we expect to submit this year.” Phase 1 Clinical Trial Design The primary objective of this Phase 1 double-blind, randomized, placebo-controlled study is to evaluate the pharmacokinetics of aficamten following administration of single ascending doses and multiple doses in 70 healthy Japanese and Caucasian participants. The secondary objective is to evaluate the safety and tolerability of aficamten in healthy Japanese and Caucasian participants. The study will enroll four cohorts including three single-ascending cohorts and one multiple dose cohort. Cohorts 1, 2 and 3 will enroll 10 Japanese participants and 10 Caucasian participants each, randomized on an 8:2 basis to receive single-ascending doses of aficamten (5 mg, 10 mg and 20 mg, respectively) or placebo. Enrollment of Cohort 2 and Cohort 3 will commence upon evaluation of the safety of the preceding Cohort. Following the completion of the single ascending dose cohorts, Cohort 4 will enroll 10 healthy Japanese participants randomized on an 8:2 basis to receive single doses of aficamten (5 mg) or placebo, once daily for 14 days. About Aficamten Aficamten is an investigational selective, small molecule cardiac myosin inhibitor discovered following an extensive chemical optimization program that was conducted with careful attention to therapeutic index and pharmacokinetic properties and as may translate into next-in-class potential in clinical development. Aficamten was designed to reduce the number of active actin-myosin cross bridges during each cardiac cycle and consequently suppress the myocardial hypercontractility that is associated with hypertrophic cardiomyopathy (HCM). In preclinical models, aficamten reduced myocardial contractility by binding directly to cardiac myosin at a distinct and selective allosteric binding site, thereby preventing myosin from entering a force producing state. The development program for aficamten is assessing its potential as a treatment that improves exercise capacity and relieves symptoms in patients with HCM as well as its potential long-term effects on cardiac structure and function. Aficamten was evaluated in SEQUOIA-HCM (Safety, Efficacy, and Quantitative Understanding of Obstruction Impact of Aficamten in HCM), a positive pivotal Phase 3 clinical trial in patients with symptomatic obstructive hypertrophic cardiomyopathy (HCM). Aficamten received Breakthrough Therapy Designation for the treatment of symptomatic obstructive HCM from the U.S. Food & Drug Administration (FDA) as well as the National Medical Products Administration (NMPA) in China. Cytokinetics expects to submit a New Drug Application (NDA) to the FDA in Q3 2024 and a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) in Q4 2024. Aficamten is also currently being evaluated in MAPLE-HCM, a Phase 3 clinical trial of aficamten as monotherapy compared to metoprolol as monotherapy in patients with obstructive HCM, ACACIA-HCM, a Phase 3 clinical trial of aficamten in patients with non-obstructive HCM, and CEDAR-HCM, a clinical trial of aficamten in a pediatric population with obstructive HCM, and FOREST-HCM, an open-label extension clinical study of aficamten in patients with HCM. About Cytokinetics Cytokinetics is a late-stage, specialty cardiovascular biopharmaceutical company focused on discovering, developing and commercializing first-in-class muscle activators and next-in-class muscle inhibitors as potential treatments for debilitating diseases in which cardiac muscle performance is compromised. As a leader in muscle biology and the mechanics of muscle performance, the company is developing small molecule drug candidates specifically engineered to impact myocardial muscle function and contractility. Cytokinetics is preparing regulatory submissions for aficamten, its next-in-class cardiac myosin inhibitor, following positive results from SEQUOIA-HCM, the pivotal Phase 3 clinical trial in obstructive hypertrophic cardiomyopathy. Cytokinetics is also developing omecamtiv mecarbil, a cardiac muscle activator, in patients with heart failure. Additionally, Cytokinetics is developing CK-586, a cardiac myosin inhibitor with a mechanism of action distinct from aficamten, for the potential treatment of HFpEF, and CK-136, a cardiac troponin activator for the potential treatment HFrEF and other types of heart failure, such as right ventricular failure resulting from impaired cardiac contractility. Cytokinetics continues its longstanding history of pioneering innovation in muscle biology and related pharmacology focused to diseases of muscle dysfunction and conditions of muscle weakness. For additional information about Cytokinetics, visit www.cytokinetics.com and follow us on X, LinkedIn, Facebook and YouTube. Forward-Looking Statements This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the “Act”). Cytokinetics disclaims any intent or obligation to update these forward-looking statements and claims the protection of the Act’s Safe Harbor for forward-looking statements. Examples of such statements include, but are not limited to, statements express or implied relating to the properties or potential benefits of aficamten or any of our other drug candidates, our ability to file a new drug application for aficamten with FDA in third quarter 2024, our ability to file a marketing authorization application for aficamten with EMA in the fourth quarter 2024, our ability to obtain regulatory approval for aficamten for the treatment of obstructive hypertrophic cardiomyopathy or any other indication from FDA or any other regulatory body in the United States or abroad, and the labeling or post-marketing obligations that may be required by FDA or any other regulatory body in the United States or abroad as a condition to regulatory approval. Such statements are based on management’s current expectations, but actual results may differ materially due to various risks and uncertainties, including, but not limited to the risks related to Cytokinetics’ business outlines in Cytokinetics’ filings with the Securities and Exchange Commission. Forward-looking statements are not guarantees of future performance, and Cytokinetics’ actual results of operations, financial condition and liquidity, and the development of the industry in which it operates, may differ materially from the forward-looking statements contained in this press release. Any forward-looking statements that Cytokinetics makes in this press release speak only as of the date of this press release. Cytokinetics assumes no obligation to update its forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release. Contact:Cytokinetics Diane WeiserSenior Vice President, Corporate Affairs(415) 290-7757
Viz.ai Collaborates with Hypertrophic Cardiomyopathy Association to Improve Care for Hypertrophic Cardiomyopathy Patients
SAN FRANCISCO–(BUSINESS WIRE)–Viz.ai, the leader in AI-powered disease detection and intelligent care coordination, today announced a collaboration with the Hypertrophic Cardiomyopathy Association (HCMA), the preeminent organization improving the lives of those with hypertrophic cardiomyopathy (HCM), to support, educate and advance research for HCM. “We’re thrilled to collaborate with the HCMA […]
Anteris Provides Update on DurAVR™ THV Valve-in-Valve Experience Presented at New York Valves 2024
DurAVR ViV restores similar aortic valve gradients to initial post-surgical results June 13, 2024 06:00 AM Eastern Daylight Time BRISBANE, Australia & EAGAN, Minn.–(BUSINESS WIRE)–Anteris Technologies Ltd (ASX: AVR), a structural heart company developing DurAVR™ THV, a new class of TAVR and the world’s only balloon-expandable, single-piece biomimetic aortic replacement […]
ABE Technology Introduces AI-Enhanced Cardiac Imaging System ‘CardioVision’
HONG KONG–(BUSINESS WIRE)–ABE Technology has announced the launch of its latest innovation, CardioVision, an AI-powered cardiac imaging system designed to improve the efficiency and accuracy of cardiac diagnostics. This new system integrates advanced artificial intelligence to streamline imaging processes and enhance patient care. CardioVision: Enhancing Cardiac Diagnostics CardioVision utilizes advanced […]