SUNNYVALE, Calif., June 07, 2024 (GLOBE NEWSWIRE) — BioCardia®, Inc. [Nasdaq: BCDA], a company focused on cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary diseases, today announces that the Unites States Patent Office has granted Patent No: 11,986,611 titled “Radial and Transendocardial Delivery Catheter,” with a patent term that will expire in 2036. The present invention relates to medical methods and systems suitable for substance delivery to the heart via a radial artery and for the intracardiac delivery of cellular aggregates and other agglomerated materials. Radial artery delivery is a means by which cardiac catheters are advanced through a blood vessel in a patient’s wrist to treat the heart. This approach has significant advantages for patients, enabling them to leave the hospital soon after the procedure with a band aid on their wrist and their arm in a simple sling, allowing them to immediately return to their active lives. In addition, a radial approach permits hospitals and other care centers to greatly reduce costs by eliminating the need for an overnight stay by the patient. For these reasons, trans-radial access is becoming the default approach in many cardiac centers worldwide. Enabling radial access has enormous potential advantages for biotherapeutic delivery to the heart. BioCardia’s Helix system, used in our ongoing clinical trials, is the only known system with patented designs that can enable radial transendocardial biotherapeutic delivery. The issued patent claims further protect this approach and add value to both BioCardia’s therapeutic programs and those of our biotherapeutic delivery partners. Therapeutic cell aggregates have the potential advantage over single cell suspensions by enhancing retention in the heart to maximize therapeutic benefit. However, the delivery of cell aggregates carries greater risks of potentially life-threatening strokes should they leak into the ventricular chamber. As 20% of the blood in the heart chamber goes to the brain, 20% of any cell or cell aggregates released in the heart are expected to obstruct the first cerebral artery that is too small to allow the cell or cell aggregate to pass. The larger the cell aggregate, the larger the vessel they are capable of obstructing, with greater risk of a significant life-threatening stroke. BioCardia’s delivery systems are designed to prevent leakage of therapeutic agents into the ventricular chamber by providing stable and safe engagement to the heart tissue during the delivery process. The issued patent claims further protect this design and add value to both BioCardia’s therapeutic programs and those of our biotherapeutic delivery partners. “Our minimally invasive biotherapeutic delivery platforms enable the successful development of cell and gene-based therapies for the heart,” said Dr. Peter Altman, BioCardia CEO. “The Helix platform underlies BioCardia’s cell therapy clinical programs and this recent patent issuance provides additional protection to our technology and product offerings in the United States for at least another dozen years. This is just one of many patent applications we are advancing to protect our value creation for the benefit of shareholders, which in turn enables these advances to be supported for expected benefit to millions of patients.” About BioCardia® BioCardia, Inc., headquartered in Sunnyvale, California, is developing cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary diseases. CardiAMP™ autologous and CardiALLO allogeneic cell therapies are the Company’s biotherapeutic platforms in three clinical stage product candidates in development. BioCardia also partners with other biotherapeutic companies to provide its delivery systems and development support to their programs. For more information visit: www.BioCardia.com. Forward Looking Statements: This press release contains forward-looking statements that are subject to many risks and uncertainties. Forward-looking statements include, among other things, references to the Company’s investigational product candidates, the benefits and risks of cell aggregates, the benefits of radial artery access, the level and duration of patent protection that the Company’s patents provide to its product candidates, and the expected safety of BioCardia’s delivery device designs. These forward-looking statements are made as of the date of this press release, and BioCardia assumes no obligation to update the forward-looking statements. We may use terms such as “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or other words that convey the uncertainty of future events or outcomes to identify these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained herein, we caution you that forward-looking statements are not guarantees of future performance and that our actual results may differ materially from the forward-looking statements contained in this press release. As a result of these factors, we cannot assure you that the forward-looking statements in this press release will prove to be accurate. Additional factors that could materially affect actual results can be found in BioCardia’s Form 10-K filed with the Securities and Exchange Commission on March 27, 2024, under the caption titled “Risk Factors” And in its subsequently filed Quarterly Reports on Form 10-Q. BioCardia expressly disclaims any intent or obligation to update these forward-looking statements, except as required by law.
Coronary/Structural Heart
University Hospitals Now Performing Robotic Heart Bypass Surgeries
CLEVELAND – A new, robotic approach to heart bypass surgery is now being offered to patients at University Hospitals (UH) with great success. Two UH Harrington Heart & Vascular Institute providers, Dr. Kelsey Gray and Dr. Pablo Ruda Vega, are currently the only surgeons in Ohio performing this specific type […]
Hypertrophic Cardiomyopathy 101
What every student-athlete should knowMISSION, Kan., June 6, 2024 /PRNewswire/ — (Family Features) You may find it difficult to wrap your mind around the idea of an energetic student-athlete with a cardiac diagnosis. Heart conditions may be more often associated with older individuals, but you might be surprised to learn hypertrophic cardiomyopathy is the most common condition responsible for sudden cardiac death in young athletes. In fact, it’s the cause of 40% of sudden cardiac death cases.
Continue Reading
Photo courtesy of Shutterstock
Photo courtesy of Shutterstock
It’s estimated 1 in every 500 adults living in the United States has hypertrophic cardiomyopathy, according to the American Heart Association, but a significant percentage are undiagnosed. More than 80% of individuals who experience this condition show no signs or symptoms before sudden cardiac death. While sudden cardiac death is rare, it can occur during exercise or in its aftermath. That’s why it’s important for student-athletes and their loved ones to learn more about this condition and talk to a doctor about their risk.
With proper knowledge and the support of a skilled care team, it’s possible to manage hypertrophic cardiomyopathy with heart-healthy actions to prevent complications or worsening cardiovascular conditions like atrial fibrillation (a quivering or irregular heartbeat), stroke or heart failure. Hypertrophic cardiomyopathy awareness and education for athletes by the American Heart Association is made possible in part by a grant from the Bristol Myers Squibb Foundation.
What is hypertrophic cardiomyopathy?Hypertrophic cardiomyopathy is the most common form of inherited heart disease and can affect people of any age. It’s defined by thickening and stiffening of the walls of the heart. The heart’s chambers cannot fill up or pump blood out adequately, so the heart is unable to function normally.There are different types of this condition. Most people have a form of the disease in which the wall that separates the two bottom chambers of the heart (the septum) becomes enlarged and restricts blood flow out of the heart (obstructive hypertrophic cardiomyopathy).However, sometimes hypertrophic cardiomyopathy occurs without significant blocking of blood flow (nonobstructive hypertrophic cardiomyopathy). The heart’s main pumping chamber is still thickened and may become increasingly stiff, reducing the amount of blood taken in then pumped out to the body with each heartbeat.What are possible symptoms?Symptoms can include:
shortness of breath
chest pain
heart palpitations
fatigue
The severity of symptoms can vary, but if you experience them or if you have a family history of hypertrophic cardiomyopathy or sudden cardiac death, it may be a good idea to speak to your doctor about whether you have this condition.For some people, symptoms can get worse and new symptoms can appear over time, resulting in people dealing with harsher effects and a diminished ability to do the activities they love. This decrease in functions can be one of the most challenging aspects of the disease. Keeping your health care team aware of any new or changing symptoms allows them to work with you to develop a plan to manage these symptoms and reduce their impact.How is hypertrophic cardiomyopathy diagnosed?Medical history, family history, a physical exam and diagnostic test results all factor into a diagnosis. A common diagnostic test is an echocardiogram that assesses the thickness of the heart muscle and observes blood flow from the heart.If anyone in your family has been diagnosed with hypertrophic cardiomyopathy, other heart diseases or has been told they had thick heart walls, you should share that information with your doctor and discuss the need for genetic testing. Because this condition is hereditary, first-degree relatives, which include siblings and parents, should be checked.Learn more at heart.org/HCMStudentAthlete.Photos courtesy of ShutterstockMichael French[email protected]1-888-824-3337editors.familyfeatures.comAbout Family Features Editorial SyndicateA leading source for high-quality food, lifestyle and home and garden content, Family Features provides readers with topically and seasonally relevant tips, takeaways, information, recipes, videos, infographics and more. Find additional articles and information at Culinary.net and eLivingToday.com.SOURCE Family Features Editorial Syndicate
MiRus Siegel™ TAVR: First in Human Results Presented at New York Valves
Five sequential patients with severe, symptomatic aortic stenosis (AS) were treated at the Instituto Nacional Del Torax in Santiago, Chile by Drs. C. Dauvergne, J. Sandoval and P. Yadav. Three patients had bicuspid aortic valves and two were tri-leaflet. Three patients had peripheral arterial disease with vascular access < 5.5 mm. There was no mortality or stroke at 30 days and no patients required a permanent pacemaker (PPM) or suffered vascular complications. At 30 days, the mean echo gradient was 6.7 mmHg; four of the five patients had no peri-valvular leak (PVL) and one bicuspid patient had trace PVL. "The ease of use and hemodynamics were impressive" commented Pradeep K. Yadav, MD. "On the very first case, we comfortably achieved a deployment with 90% aortic and 10% ventricular positioning. The lack of foreshortening is very helpful in precise deployment every time, a feature that implanters will love. Also the frame strength and virtually no recoil, allows cylindrical valve expansion with no waist even in complex bicuspid patients, which contributes to excellent hemodynamics and hopefully durability." The Siegel valve represents several firsts in TAVR: 8 French delivery sheath allowing less invasive procedures and broader patient access, particularly for women; the only Nickel-free THV allowing treatment of the 20% of Americans suffering from Nickel allergies; precise delivery due to lack of foreshortening and intrinsic commissural alignment; dry porcine pericardial leaflets with anti-calcification treatment and with the valve pre-mounted on the balloon.The combination of low delivery system profile and excellent hemodynamics is made feasible by the unique properties of the Rhenium alloys pioneered by MiRus including high yield strength, fatigue resistance and minimal recoil."This initial data is striking and potentially heralds a new age for TAVR," stated Vinod H. Thourani MD, Marcus Chairman of Cardiovascular Surgery and the Marcus Valve Center, Piedmont Heart Institute. "The ability to treat such complex patients with an 8 French system and without Nickel exposure should make TAVR safer and more broadly accessible. From a surgical viewpoint, the very low pressure gradients and low PVL are critically important to implanters and our patients. We are truly on the precipice of surgical-like outcomes with the Siegel THV!!"About MiRus, LLC.MiRus is a life sciences company headquartered in Marietta, Georgia that has developed and is commercializing proprietary novel biomaterials, implants and procedural solutions for the treatment of spine, orthopaedic and structural heart disease. Inspired by the pioneering material science of NASA for rocket engines, MiRus has created Rhenium based medical alloys that are transforming medicine by making surgeries less invasive and implants safer and more durable. Find out more information about MiRus at www.mirusmed.com. Statements made in this press release that look forward in time or that express beliefs, expectations or hopes regarding future occurrences or anticipated outcomes are forward-looking statements. A number of risks and uncertainties such as risks associated with product development and commercialization efforts, expected timing or results of any clinical trials, ultimate clinical outcome and perceived or actual advantages of the Company's products, market and physician acceptance of the products, intellectual property protection, and competitive offerings could cause actual events to adversely differ from the expectations indicated in these forward looking statements. The Siegel TAVR system is an investigational device and not FDA approved.* MiRus® , Siegel™ are all trademarks of MiRus, LLC. Contact:Pam CowartVP of Clinical Affairs[email protected]770-861-4804SOURCE MiRus
ECHO IQ TECHNOLOGY CHAMPIONED IN INDEPENDENT SCIENTIFIC REVIEW
Echo IQ’s EchoSolv-AS decision-support software for cardiology delivers significant potential benefits to hospital networks according to independent scientific presentation at prestigious New York Valves scientific conference.
NEW YORK, June 6, 2024 /PRNewswire/ — At the Structural Heart Summit New York Valves earlier today, Dr. Pedro Covas (Baylor Scott & White, The Heart Hospital Plano TX) delivered the scientific presentation: AI-Powered Cardiac Ultrasound Improves Identification of High-Risk Aortic Stenosis (Echo IQ).
Dr. Covas highlighted the unmet need for an AI system for aortic stenosis – a form of heart valve disease characterised by high rates of mortality when untreated. He revealed that the condition is underdiagnosed and undertreated worldwide, partly due to the complexity in the diagnosis of severe aortic stenosis (including low-flow states), and that there is a need for assistance to improve rates of diagnosis. As backed up by the research presented by Dr. Covas, Echo IQ uses artificial intelligence to aide in identifying a high-risk phenotype of aortic stenosis, to ultimately help with timely diagnosis and treatment.
LEARN HOW ECHO IQ USES AI TO SUPPORT ENHANCED DISEASE DETECTION:
The independent research presented by Dr. Covas considered whether artificial intelligence could improve accuracy and reproducibility of diagnosis of severe aortic stenosis in a real-world hospital setting. To test this, Echo IQ’s technology (EchoSolv) was applied to the echocardiographic report data of one of Baylor Scott & White’s leading academic heart hospitals over a 7-month period with a subset of the assessments adjudicated via comprehensive image review by expert cardiographers.
The findings showed EchoSolv to be successful in identifying more patients at risk of aortic stenosis than human-only diagnosis. Specifically,
EchoSolv accurately identified 15% more patients with severe aortic stenosis than human-only diagnosis
Where initial underdiagnosis occurred, patients were found to have a “low flow” state of disease in 30% of cases (subsequently identified by EchoSolv)
The research concluded that:
Echo IQ’s technology can automatically identify aortic stenosis patients at-risk using only echocardiographic measurement data.
Echo IQ’s technology has the potential to improve diagnostic rates of severe aortic stenosis, particularly in low flow states.
There are important implications for less specialized cardiology centers, where echocardiographers are likely to benefit the most from automated diagnosis of severe AS.
Echo IQ Chief Medical Advisor, Professor David Playford said: “This kind of independent research demonstrates exactly how Echo IQ’s artificial intelligence can help healthcare professionals identify patients with severe cases of aortic stenosis as well as those with significant risk of disease. Diagnosing aortic stenosis accurately, and in a timely fashion, is extremely complex and the findings shared by Dr. Covas show clearly how Echo IQ’s artificial intelligence can help improve clinical performance.”
About Echo IQ
Echo IQ uses artificial intelligence for decision support in structural heart disease. The company’s first fully AI-enabled solution has been submitted for final FDA clearance. The company is headquartered in Sydney, Australia and is publicly listed on the Australian Securities Exchange (ASX:EIQ). Don Fowler is President, Echo IQ USA, and is based in Austin, TX.
SOURCE Echo IQ Limited
Prevencio Selected for Prestigious MedTech Innovator 2024 Accelerator Cohort
KIRKLAND, Wash.–(BUSINESS WIRE)–Prevencio, Inc., a leader in AI-powered blood tests for cardiovascular diagnostics, has been selected to join the 2024 Accelerator Cohort of MedTech Innovator, the world’s largest accelerator for medical technology companies. This honor places Prevencio among the top 5% of 1,300 applicants, reflecting the company’s innovative approach to […]
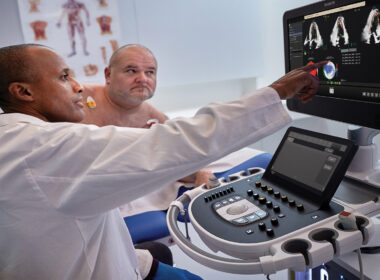
Deep integration of proven AI tools into Philips cardiovascular ultrasound systems to better diagnose more cardiac disease patients
June 4, 2024 New FDA-cleared AI-enabled applications include the industry’s first automated tool for segmental wall motion scoring of the heart to quickly and objectively identify disorders including coronary artery disease and cardio-oncology issues in secondsFirst fully automated 3D quantification of mitral regurgitation (MR) volumes* designed to provide reproducible, efficient analysis helps clinicians make better-informed decisions for patients with heart valve disease Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, announced its next-generation AI-enabled cardiovascular ultrasound platform to help speed up cardiac ultrasound analysis with proven AI technology and reduce the burden on echocardiography labs. Integrated into the company’s EPIQ CVx and Affiniti CVx ultrasound systems, the new FDA 510(k) cleared AI applications significantly advance Philips’ cardiovascular imaging and diagnosis solutions, automating measurements and speeding workflows to increase productivity. Heart failure is a rapidly growing health issue that affects an estimated 64 million people worldwide [1]. It’s also associated with high mortality and poor quality of life, and is a substantial burden on healthcare systems globally [2]. As the most common and least invasive way to check the structure and function of the heart, cardiovascular ultrasound has played a key role in diagnosing cardiac disease earlier. “As clinical cases get more complex and patient volumes increase, we read hundreds of echocardiography exams daily with thousands of data points,” said Roberto Lang, MD, Director of the Noninvasive Cardiac Imaging Lab, University of Chicago Medicine, USA. “With the integration of AI into echocardiography solutions, we can now automate some of the steps to support clinicians’ decision-making, allowing them to detect, diagnose, and monitor various cardiac conditions with greater confidence and efficiency in seconds.” Dr. Lang will join other clinicians to share the results of a new scientific abstract being presented at the American Society of Echocardiography (ASE2024) annual meeting (June 14 – 16, Portland, US), demonstrating how first-of-kind AI algorithms co-developed with Philips provide highly accurate detection of regional wall motion abnormalities (RWMA) on echocardiography. RWMAs can be an independent indicator of adverse cardiovascular events and death in patients with cardiovascular diseases like myocardial infarction (MI) and congenital heart disease. Automated machine learning-based assessment of RWMA has the potential to improve the efficiency of all readers. “An advantage of AI methods over conventional visual analysis is that it can be performed in seconds, providing rapid and accurate information to help augment expert reads by quickly highlighting areas of concern for RWMA, improving the ease and efficiency of interpretation,” Lang added. “By harnessing the power of AI into our echocardiography solutions, we empower clinicians with enhanced diagnostic capabilities, to ultimately improve patient care and outcomes in the management of coronary and valvular disease, while enhancing overall efficiency in cardiac practice,” said David Handler, VP and Business Leader for Global Cardiovascular Ultrasound at Philips. “For patients, this means consistent image interpretation which can lead to fewer re-scans, shorter and more effective interventional procedures, and potentially faster recovery times.” Integration of proven AI applications from Philips DiA Imaging Analysis Trained on anonymized patient data sets from real-life clinical environments, the AI features integrated across Philips cardiovascular ultrasound systems help improve the quality and reproducibility of cardiac imaging and enhance operator and departmental efficiency. These include FDA-cleared and CE-marked software solutions from DiA Imaging Analysis, a Philips company. Together, these AI features automate how users interpret ultrasound images, so clinicians with varying levels of ultrasound experience can automatically analyze images with increased speed, efficiency, and accuracy in real time. In addition to integrating these latest advanced AI features into the company’s cardiovascular ultrasound systems, Philips’ new mini ultrasound transducer X11 4t, introduced earlier this year, is fully compatible with the EPIQ CVx and Affiniti CVx systems. The innovative transducer allows interventional cardiologists to provide enhanced care to a wider range of patients, including pediatric patients as small as 5kg in weight. Through breakthrough innovation and collaborations with technology leaders including NVIDIA**, Philips continues to rapidly integrate AI into its cardiovascular ultrasound portfolio to help improve efficiency and productivity and mitigate staff shortages while delivering high-quality cardiology care to an increasing number of patients. These third-party solutions complement Philips’ proprietary AI-enabled solutions, integrating AI to enhance diagnostic confidence and decision-making. For more information, join Philips in Booth #441 at ASE2024 and visit Philips Cardiovascular Ultrasound for the latest AI-powered echocardiography applications. *Clinical performance and safety have not been established for some features which have 510(k) pending. Not available for sale in the USA.**NVIDIA MONAI for medical imaging AI and NVIDIA Holoscan for building software-defined medical devices. [1] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10398425/ [2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10398425/ For further information, please contact: Kathy O’ReillyPhilips Global External RelationsTel.: +1 978 221 8919E-mail : kathy.oreilly@philips.com About Royal Philips Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring, and enterprise informatics, as well as in personal health. Philips generated 2023 sales of EUR 18.2 billion and employs approximately 69,100 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Attachments
Orchestra BioMed Announces Further Details on In-Person R&D Day in New York Featuring Key Opinion Leaders to Discuss AVIM Therapy Program in Hypertensive Pacemaker Patients on June 11, 2024
NEW HOPE, Pa., June 04, 2024 (GLOBE NEWSWIRE) — Orchestra BioMed Holdings, Inc. (Nasdaq: OBIO, “Orchestra BioMed” or the “Company”), a biomedical company accelerating high-impact technologies to patients through risk-reward sharing partnerships, today announced further details on its in-person R&D day at the Lotte New York Palace Hotel on Tuesday, June 11, 2024, at 10:00 AM ET. To register, click here. The R&D day will feature in-depth presentations focused on the Company’s lead program, atrioventricular interval modulation (AVIM) therapy, including: The high unmet need for treatment of uncontrolled hypertension in pacemaker-indicated patients and other older high-risk patientsThe AVIM therapy mechanism of action and supporting clinical and non-clinical mechanistic dataThe robust existing body of clinical evidence from the MODERATO I and II studiesDesign of and rationale for the BACKBEAT global pivotal study In addition to Orchestra BioMed management presentations, the event will include esteemed key opinion leading physicians: David Kandzari, M.D., FACC, FSCAI, Chief of the Piedmont Heart Institute and Cardiovascular Service Line; Director, Interventional Cardiology of the Piedmont Heart Institute; Chief Scientific Officer for Piedmont Healthcare in Atlanta, Georgia; and co-principal investigator on the BACKBEAT global pivotal study Dr. Kandzari specializes in cardiovascular disease, peripheral arterial disease and interventional cardiology. Board certified by the American Board of Internal Medicine and its subspecialty Board of Cardiovascular Disease and Board of Interventional Cardiology, Dr. Kandzari has held national and international leadership roles in clinical trials in cardiovascular disease and has participated in national and international program committees in cardiology. He has authored and coauthored more than 400 studies, book chapters and scientific reviews, and has delivered more than 800 lectures, both nationally and internationally, on a variety of issues related to both interventional and general cardiology. Dr. Kandzari has been consecutively voted as one of Atlanta’s Top Doctors by Atlanta Magazine from 2011 to 2024 and is peer-nominated in the top 1% of cardiologists by U.S News and World Report. Vivek Reddy, M.D., Director of Cardiac Arrhythmia Services for The Mount Sinai Hospital and the Mount Sinai Health System; Leona M. and Harry B. Helmsley Charitable Trust Professor of Medicine in Cardiac Electrophysiology at Icahn School of Medicine at Mount Sinai; and Clinical Steering Committee Advisor for the BACKBEAT global pivotal study Dr. Reddy is one of the nation’s premier cardiac electrophysiologists, leading a team of physician-scientists who are developing and testing advanced therapies for cardiac arrhythmias – including atrial fibrillation and ventricular tachycardias, the most common cause of sudden cardiac death. Dr. Reddy’s pioneering clinical research is changing the way cardiac patients are treated and cured. Under his leadership, Mount Sinai is the lead site on several multinational clinical trials exploring new arrhythmia procedures and technologies. In 2014, Dr. Reddy implanted the world’s first miniature leadless pacemaker in a patient’s heart in the United States at The Mount Sinai Hospital. A live question and answer session will follow formal presentations. About Orchestra BioMed Orchestra BioMed (Nasdaq: OBIO) is a biomedical innovation company accelerating high-impact technologies to patients through risk-reward sharing partnerships with leading medical device companies. Orchestra BioMed’s partnership-enabled business model focuses on forging strategic collaborations with leading medical device companies to drive successful global commercialization of products it develops. Orchestra BioMed’s lead product candidate is atrioventricular interval modulation (AVIM) therapy (also known as BackBeat Cardiac Neuromodulation Therapy (CNT™)) for the treatment of hypertension, a significant risk factor for death worldwide. Orchestra BioMed is also developing Virtue® Sirolimus AngioInfusion™ Balloon (SAB) for the treatment of atherosclerotic artery disease, the leading cause of mortality worldwide. Orchestra BioMed has a strategic collaboration with Medtronic, one of the largest medical device companies in the world, for the development and commercialization of AVIM therapy for the treatment of hypertension in pacemaker-indicated patients, and a strategic partnership with Terumo, a global leader in medical technology, for the development and commercialization of Virtue SAB for the treatment of artery disease. For further information about Orchestra BioMed, please visit www.orchestrabiomed.com, and follow us on LinkedIn. References to Websites and Social Media Platforms References to information included on, or accessible through, websites and social media platforms do not constitute incorporation by reference of the information contained at or available through such websites or social media platforms, and you should not consider such information to be part of this press release. About Hypertension and the Risk of High Blood Pressure in the Pacemaker Population Hypertension (“HTN”) is characterized by elevated blood pressure, which increases the force of blood pushing against blood vessels, requiring the heart to work harder and consume more oxygen. HTN accelerates the progression of atherosclerosis and leads to increased risk of major cardiac events like heart attack, heart failure, kidney disease and other end organ damage. HTN is the leading global risk factor for death, affecting an estimated 1.28 billion adults worldwide. In the United States, 122 million adults, or approximately 47% of all adults, are estimated to have HTN. While many patients do not notice high blood pressure, cardiovascular risk doubles for every 10-mmHg increase in systolic blood pressure and the mortality rate doubles with an increase of 20 mmHg in systolic blood pressure.1 It is estimated that more than 70% of the approximately 1.1 million people globally who are implanted with cardiac pacemakers each year are also diagnosed with HTN. Based on updated American College of Cardiology/American Heart Association guidelines, we estimate that an even higher percentage (approximately 80%) of U.S. patients who are indicated for a pacemaker implant have HTN. Pacemaker patients tend to be elderly and are more likely to suffer from co-morbidities, such as atherosclerosis, hyperlipidemia, diabetes mellitus and chronic kidney disease, and harder to treat effectively with medical therapy for many reasons, including co-morbidities and a high prevalence of isolated systolic HTN. About AVIM Therapy (BackBeat CNT™) AVIM therapy, also known as BackBeat CNT™, is an investigational therapy compatible with standard dual-chamber pacemakers designed to substantially and persistently lower blood pressure. It has been evaluated in pilot studies in patients with hypertension who are also indicated for a pacemaker. MODERATO II, a double-blind, randomized, pilot study, showed that patients treated with AVIM therapy experienced net reductions of 8.1 mmHg in 24-hour ambulatory systolic blood pressure (aSBP) and 12.3 mmHg in office systolic blood pressure (oSBP) at six months when compared to control patients. The IDE approved BACKBEAT (BradycArdia paCemaKer with atrioventricular interval modulation for Blood prEssure treAtmenT) global pivotal study will further evaluate the safety and efficacy of AVIM therapy in lowering blood pressure in a similar target population of patients who have been indicated for, and recently implanted with, a dual-chamber cardiac pacemaker. References: 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al., Heart Disease and Stroke Statistics – 2017 Update: A Report from the American Heart Association. Circulation. 2017; 135: e146. Investor Contact:Bob YedidLifeSci Advisors(516) 428-8577Bob@lifesciadvisors.com Media Contact:Kelsey Kirk-EllisOrchestra BioMed(484) 682-4892Kkirkellis@orchestrabiomed.com
BridgeBio Announces Durable Month 12 and 18 Phase 2 Cohort 5 Results of Oral Infigratinib in Achondroplasia, and First Participant Consented in ACCEL for Hypochondroplasia
– In Cohort 5 of PROPEL 2 (0.25 mg/kg/day), oral treatment with infigratinib resulted in a statistically significant and sustained increase in annualized height velocity (AHV), with a mean change from baseline of +2.51cm/yr at Month 12, and +2.50 cm/yr at Month 18 (p=0.0015) – At Month 18, there was a statistically significant improvement in body proportionality (p-value of 0.001). The mean upper to lower body segment ratio was 1.88 at Month 18, as compared to 2.02 at baseline – Infigratinib continues to be well-tolerated as a single daily oral therapy with no adverse events (AEs) assessed as treatment-related in any participant in Cohort 5 – PROPEL 3, the global Phase 3 registrational study of infigratinib in achondroplasia, continues to enroll on schedule, with completion estimated by end of 2024 – BridgeBio announces first child consented in ACCEL, the observational run-in study for infigratinib in children living with hypochondroplasia, a skeletal dysplasia closely related to achondroplasia and similarly driven by FGFR3 gain-of-function variants PALO ALTO, Calif., June 04, 2024 (GLOBE NEWSWIRE) — BridgeBio Pharma, Inc. (Nasdaq: BBIO) (BridgeBio), a commercial-stage biopharmaceutical company focused on genetic diseases, today announced sustained positive results from PROPEL 2, a Phase 2 trial of the investigational therapy infigratinib in children with achondroplasia, demonstrating continued potential best-in-class efficacy and an encouraging safety profile. Infigratinib is an oral small molecule designed to inhibit FGFR3 signaling and target achondroplasia and hypochondroplasia at their source. BridgeBio will also host an investor call on June 4, 2024, at 8:00 am ET with Ravi Savarirayan, M.D., Ph.D., of Murdoch Children’s Research Institute in Melbourne, Australia, and the global lead investigator for PROPEL 2, to discuss the results from the Phase 2 study. To date, key results from the Cohort 5 dose escalation cohort in PROPEL 2 trial include: Sustained and statistically significant mean increase in AHV of +2.51cm/year from baseline at 12 months, and +2.50 cm/yr at 18 months (p=0.0015)Statistically significant improvement in body proportionality (mean upper to lower body segment ratio), from 2.02 at baseline to 1.88 at Month 18 (mean change from baseline, p=0.001)A continued well-tolerated safety profile, with no treatment-related adverse events assessed as related to infigratinib “These data indicate that treatment with infigratinib is continuing to show increased growth velocity and improvements in body proportionality in children with achondroplasia. This is encouraging and suggests that infigratinib has the potential to enhance functionality for people living with achondroplasia in addition to increasing growth. We hope to see these improvements reflected in the ongoing PROPEL 3 pivotal study that will build toward providing a safe and effective oral therapy to those in the achondroplasia community who are seeking treatment,” said Dr. Savarirayan. PROPEL 3, the global Phase 3 registrational study of infigratinib in achondroplasia, continues to enroll on schedule, with completion of enrollment anticipated by the end of the year. Given the promising results from PROPEL 2, BridgeBio is committed to expanding the FGFR3-related skeletal dysplasias franchise for infigratinib by accelerating development in hypochondroplasia. Positive interactions with both the U.S. FDA and EMA support development in children with hypochondroplasia, with a small open-label Phase 2 portion testing a single dose of 0.25 mg/kg/day, leading into a double-blinded, placebo-controlled Phase 3 study. ACCEL, the observational lead-in program for hypochondroplasia, was initiated with the first participant consented in May 2024. The interventional program, ACCEL 2/3, will be a global Phase 2/3 multicenter, single-dose study, to evaluate the efficacy and safety of 0.25mg/kg/day of infigratinib in children living with hypochondroplasia. The open-label Phase 2 portion in children aged 5 to 11 years old will be followed by a pivotal Phase 3, one-year, 2:1 randomized, double-blinded, placebo-controlled study in children aged 3 to < 18 years old with growth potential. In addition to changes from baseline in AHV measurements, the study will evaluate changes in other indicators of growth, body proportions, medical complications associated with hypochondroplasia, and changes in quality-of-life measures. BridgeBio has previously presented promising preclinical data for hypochondroplasia at ENDO 2023 and ASHG 2022. “We are very excited to see a persistence of response to infigratinib in linear growth. We are especially encouraged by the promising effect on body proportions, which supports infigratinib’s potential to provide benefits that could impact the lives of children with achondroplasia. These results motivate us to continue evaluating infigratinib in other FGFR-related skeletal dysplasias and genetic conditions. The initiation of our observational study in hypochondroplasia and the obtainment of FDA and EMA alignment on the interventional study underlie our excitement for the potential of infigratinib as a treatment option for children with hypochondroplasia,” said Daniela Rogoff, M.D., Ph.D., Chief Medical Officer, Skeletal Dysplasias at BridgeBio. “The journey of living with skeletal dysplasia varies from person to person, but many are impacted by functional limitations, social stigma and medical complications due to their condition and the way their bones develop. We are encouraged to see infigratinib’s potential to improve body proportionality, which could help address functional complications meaningful to people living with skeletal dysplasia. The Chandler Project values the collaborative partnership we’ve developed with BridgeBio and QED, to ensure that the community’s true needs are prioritized throughout the discovery and development process. We are also thrilled for the launch of an observational study in hypochondroplasia, a community that has been eager for further research and development of treatment options,” said Chandler Crews, founder of The Chandler Project, a patient advocacy organization based in Baltimore, MD. Information about PROPEL 3 (NCT06164951) can be found here on clinicaltrials.gov. Information about PROPEL (NCT04035811), BridgeBio’s observational lead-in study in achondroplasia for PROPEL 3 and other studies, can be found here on clinicaltrials.gov. Information about ACCEL (NCT06410976), BridgeBio’s observational lead-in study in hypochondroplasia can be found here on clinicaltrials.gov. BridgeBio is committed to exploring the potential of infigratinib on wider medical and functional impacts of achondroplasia, hypochondroplasia and other skeletal dysplasias, which hold significant unmet needs for families. Webcast InformationBridgeBio will host an investor call and simultaneous webcast to discuss the Phase 2 data at Months 12 and 18 of infigratinib in children with achondroplasia on June 4, 2024 at 8:00 am ET. A link to the webcast may be accessed from the event calendar page of BridgeBio’s website at https://investor.bridgebio.com/. A replay of the conference call and webcast will be archived on the Company’s website and will be available for at least 30 days following the event. About AchondroplasiaAchondroplasia is the most common cause of disproportionate short stature, affecting approximately 55,000 people in the United States (US) and European Union (EU), including up to 10,000 children and adolescents with open growth plates. Achondroplasia impacts overall health and quality of life, leading to medical complications such as obstructive sleep apnea, middle ear dysfunction, kyphosis, and spinal stenosis. The condition is uniformly caused by an activating variant in FGFR3. About HypochondroplasiaHypochondroplasia is also an FGFR3-associated skeletal dysplasia and is a rare condition with similar prevalence in achondroplasia. Hypochondroplasia presents with a wide spectrum of phenotypes including disproportionate short stature, mild joint laxity and macrocephaly. Currently, no treatments for hypochondroplasia are approved in the United States. About BridgeBio Pharma, Inc.BridgeBio Pharma (BridgeBio) is a commercial-stage biopharmaceutical company founded to discover, create, test and deliver transformative medicines to treat patients who suffer from genetic diseases. BridgeBio’s pipeline of development programs ranges from early science to advanced clinical trials. BridgeBio was founded in 2015 and its team of experienced drug discoverers, developers and innovators are committed to applying advances in genetic medicine to help patients as quickly as possible. For more information visit bridgebio.com and follow us on LinkedIn and Twitter. BridgeBio Pharma, Inc. Forward-Looking StatementsThis press release contains forward-looking statements. Statements in this press release may include statements that are not historical facts and are considered forward-looking within the meaning of Section 27A of the Securities Act of 1933, as amended (the Securities Act), and Section 21E of the Securities Exchange Act of 1934, as amended (the Exchange Act), which are usually identified by the use of words such as “anticipates,” “believes,” “continues,” “estimates,” “expects,” “hopes,” “intends,” “may,” “plans,” “projects,” “remains,” “seeks,” “should,” “will,” and variations of such words or similar expressions. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Exchange Act. These forward-looking statements, including statements relating to the clinical, therapeutic and market potential of our programs and product candidates, including our clinical development program for infigratinib in achondroplasia, the timing and success of our clinical development programs, the progress of our ongoing and planned clinical trials of infigratinib in achondroplasia and in hypochondroplasia , our planned interactions with regulatory authorities, the availability of data from our clinical trials of infigratinib, and the timing of these events, reflect our current views about our plans, intentions, expectations and strategies, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations and strategies as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a number of risks, uncertainties and assumptions, including, but not limited to, initial and ongoing data from our clinical trials not being indicative of final data, the design and success of ongoing and planned clinical trials, difficulties with enrollment in our clinical trials, adverse events that may be encountered in our clinical trials, the FDA or other regulatory agencies not agreeing with our regulatory approval strategies, components of our filings, such as clinical trial designs, conduct and methodologies, or the sufficiency of data submitted, potential adverse impacts due to global health emergencies, including delays in regulatory review, manufacturing and supply chain interruptions, adverse effects on healthcare systems and disruption of the global economy, the impacts of current macroeconomic and geopolitical events, including changing conditions from hostilities in Ukraine and in Israel and the Gaza Strip, increasing rates of inflation and rising interest rates, on our business operations and expectations, as well as those risks set forth in the Risk Factors section of our most recent Annual Report on Form 10-K and our other filings with the U.S. Securities and Exchange Commission. Moreover, we operate in a very competitive and rapidly changing environment in which new risks emerge from time to time. These forward-looking statements are based upon the current expectations and beliefs of our management as of the date of this press release, and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Except as required by applicable law, we assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. BridgeBio Contact:Vikram Balicontact@bridgebio.com(650)-789-8220 Medical Information Contact:MedInfo@QEDTx.com1-877-280-5655
Program Announcement for Annual ASPC Congress on CVD Prevention August 2024
Detailed Program Agendas Released for Pre-conference Course and Scientific Sessions
KINGWOOD, Texas, June 4, 2024 /PRNewswire/ — The ASPC announces details of a full program of events to be held at their annual Congress on Cardiovascular Disease Prevention, scheduled for August 1-4, 2024, at the Hyatt Regency hotel in Salt Lake City, UT.
A new pre-conference MasterClass focused on lifestyle medicine is being offered Thu., August 1 – Hot Topics in Preventive Cardiology and Lifestyle Medicine: Implications for Patient Counseling and Management. This program is being Co-Chaired by Barry Franklin, PhD, FASPC and Heather Johnson, MD, MS, FASPC. They shared, “Americans have a shorter life expectancy compared with residents of almost all other high-income countries. We also have the most expensive healthcare system in the world. It’s simply not sustainable! Accordingly, readily accessible preventive health interventions should be a top priority. This unique and timely Masterclass will address these issues – and more.”
Features of the 2024 Scientific Sessions program include diverse content with many international experts joining the program, honorees in the field of prevention, and a record number of abstracts in the poster hall!
The three ASPC honorees are:
Honorary Fellow Award: Dr. Chris Cannon, Professor of Medicine at Harvard University. His award lecture will focus on “Lipid Lowering 2024 and Beyond”.
Nanette Wenger Award: Dr. Noel Bairey Merz, Director of the Barbra Streisand Women’s Heart Center at Cedars-Sinai Heart Institute. She will present a lecture titled, “Where are We Now? – From WISE to Chest Pain Guidelines and Improving CVD Care for Women.”
Joseph Stokes III, MD Pioneer in Prevention Award: Dr. Sergio Fazio, VP/Chair, Scientific Council, Global Clinical Development at Regeneron Pharmaceuticals and the first Editor-in-Chief of the American Journal of Preventive Cardiology. Dr. Fazio’s award lecture topic is to be announced.
ASPC is looking forward to honoring these awardees during the opening session of the Congress on Friday, August 2 from 8:00 – 9:30 AM.
Throughout the weekend, the Scientific Sessions will collaborate with societies from around the globe, including:
Society of Cardiovascular Computed Tomography (SCCT)
Korean Society of Lipid and Atherosclerosis (KSoLA)
Korean Society of Cardiovascular Disease Prevention (KSCP)
European Atherosclerosis Society (EAS)
Three rousing debates will be featured during Session 7 on Saturday, August 3, from 9:00-11:00am:
Reducing Residual CVD risk: Adding Colchicine vs. Aggressive Lipid Lowering
Mike Wilkinson, MD vs. Daniel Soffer, MD
CAC/CCTA is the Imaging Modality of Choice in Refining ASCVD Risk in Primary Prevention
Seamus Whelton, MD, MPH vs. Matthew Budoff, MD, FASPC
Lp(a) in Primary Prevention is Actionable Today
Vera Bittner, MD vs. Roger Blumenthal, MD, FASPC
Detailed program agendas for both the Congress on CVD Prevention and the Lifestyle MasterClass are available at www.aspconline.org.
The ASPC’s mission is to promote the prevention of cardiovascular disease, advocate for the preservation of cardiovascular health, and disseminate high-quality, evidence-based information through the education of healthcare clinicians and their patients.
SOURCE The American Society for Preventive Cardiology