TEL AVIV, Israel, Jan. 10, 2019 /PRNewswire/ — Perflow Medical, an Israeli-based medtech company focused on next-generation neuro-interventional devices, today announced the issuance of four international patents that expand the global intellectual property (IP) coverage for the novel Stream™ Dynamic Neuro-Thrombectomy Net and Cascade™ Non-Occlusive Remodeling Net. Perflow’s growing global portfolio of eight issued […]
Neuro
MRI Interventions Receives FDA Clearance for ClearPoint® PURSUIT™ Neuro Aspiration System
IRVINE, Calif., Jan. 03, 2019 (GLOBE NEWSWIRE) — MRI Interventions, Inc. (OTCQB:MRIC), a platform neurosurgery company with products designed for navigation in procedures involving deep-brain stimulation, ablation, and gene and drug delivery today announced FDA Clearance of the ClearPoint PURSUIT Neuro Aspiration system. The PURSUIT device was designed in collaboration […]
InspireMD Announces Publication of Meta-Analysis Citing Benefits of Next Generation Mesh-Covered Carotid Stent Systems
TEL AVIV, Israel, Dec. 18, 2018 (GLOBE NEWSWIRE) — InspireMD, Inc. (NYSE American: NSPR), developer of the CGuard™ Embolic Prevention System (EPS) for the prevention of stroke caused by the treatment of carotid artery disease, today announced the publication of a meta-analysis of four clinical studies involving dual layered and […]
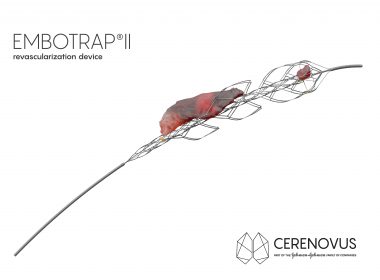
CERENOVUS LAUNCHES LARGEST GLOBAL REGISTRY TO STUDY STROKE-INDUCING BLOOD CLOTS REMOVED BY THROMBECTOMY
Company Also Receives European CE Mark Approval for Novel Stroke Technology Designed for Difficult to Extract Blood Clots IRVINE, Calif., Dec. 10, 2018 — Johnson & Johnson Medical Devices Companies* today announced that its CERENOVUS business has launched the single largest global registry, the EXCELLENT Registry, to collect and analyze […]
InspireMD Announces Positive Long-Term Safety and Efficacy Data from Ongoing CGuard™ EPS Registries at the Recent 45th Annual Symposium on Vascular and Endovascular Issues, Techniques, Horizons (VEITHsymposium)
TEL AVIV, Israel, Nov. 29, 2018 (GLOBE NEWSWIRE) — InspireMD, Inc. (NYSE American: NSPR), developer of the CGuard™ Embolic Prevention System (EPS) for the prevention of stroke caused by the treatment of carotid artery disease, today announced details from several presentations of updated CGuard™ EPS data at the recent VEITHsymposium […]
InspireMD Announces Regulatory and Reimbursement Approval of CGuard™ Embolic Prevention System in Australia
TEL AVIV, Israel, Nov. 28, 2018 (GLOBE NEWSWIRE) — InspireMD, Inc. (NYSE American: NSPR), developer of the CGuard™ Embolic Prevention System (EPS) for the prevention of stroke caused by the treatment of carotid artery disease, today announced that its novel CGuard™ Embolic Prevention System (EPS) has been granted regulatory approval […]
iSchemaView RAPID™ Integrates with Join, Allm’s Medical Communications Platform
MENLO PARK, Calif.–(BUSINESS WIRE)–iSchemaView, the worldwide leader in advanced imaging for stroke, today announced that the RAPID platform is now compatible with Join, a medical communication app developed by Allm, a multinational developer of mobile communications platforms for the healthcare industry. The integration of RAPID imaging results into the Join […]
InspireMD Announces Presentation of Preliminary Cumulative Three-Year CGuard™ EPS Safety and Efficacy Data at the Transcatheter Cardiovascular Therapeutics 2018 Conference
TEL AVIV, Israel, Sept. 27, 2018 (GLOBE NEWSWIRE) — InspireMD, Inc. (NYSE American: NSPR), developer of the CGuard™ Embolic Prevention System (EPS) for the prevention of stroke caused by the treatment of carotid artery disease, today announced that preliminary cumulative three-year follow-up safety, efficacy and stroke prevention durability data from […]
InspireMD Announces Regulatory Approval of CGuard™ Embolic Prevention System in Mexico
TEL AVIV, Israel, Sept. 24, 2018 (GLOBE NEWSWIRE) — InspireMD, Inc. (NYSE American: NSPR), developer of the CGuard™ Embolic Prevention System (EPS) for the prevention of stroke caused by the treatment of carotid artery disease, today announced that its novel CGuard™ Embolic Prevention System (EPS) has been granted regulatory approval […]
Contego Medical Receives 510(k) Clearance for the Paladin Carotid PTA Balloon System with Integrated Embolic Protection
RALEIGH, N.C., Sept. 18, 2018 /PRNewswire/ — Contego Medical announced today that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the first filter-based Integrated Embolic Protection (IEP) device, the Paladin® Carotid PTA Balloon System. Contego Medical is developing and commercializing a suite of next-generation devices that address unmet needs in […]