LOS ALTOS, Calif.–(BUSINESS WIRE)–Fist Assist Devices, LLC, a novel medical device startup based in Silicon Valley, Calif., today announced the approval of FACT (Fist Assist Clinical Trial) by the University of Chicago Medical Center Institutional Review Board (IRB). This research will evaluate the effects of using the Fist Assist® device to […]
Peripheral/Endo
Hemostemix Announces Enrollment Milestone in the ACP-01 Phase II Critical Limb Ischemia Clinical Trial
CALGARY, Alberta, April 09, 2019 (GLOBE NEWSWIRE) — Hemostemix Inc. (“Hemostemix” or the “Company”) (TSX VENTURE: HEM; OTCQB: HMTXF) is pleased to announce that it has reached an enrollment milestone in its continuing Phase II Clinical Trial for critical limb ischemia (“CLI”). Enrollment rates have increased substantially over the past […]
FEops HEARTguide™ Receives CE Mark
GENT, Belgium–(BUSINESS WIRE)–FEops, a leader in personalized predictive planning for structural heart interventions, today announced that FEops HEARTguideTM has received CE mark approval. FEops HEARTguideTM is a one-in-its-kind procedure planning environment for structural heart interventions that provides physicians unique insights to evaluate device sizing and positioning pre-operatively using novel computational modeling and simulation technology. The current release […]
SANUWAVE Receives U.S. and European Patents for Intracorporeal Shockwave Systems for Treating Blood Vessels in Cardiology and Endovascular Fields
SUWANEE, GA, April 02, 2019 (GLOBE NEWSWIRE) — via NEWMEDIAWIRE — SANUWAVE Health, Inc. (OTCQB: SNWV), an emerging medical technology company focused on the development and commercialization of noninvasive, biological response activating devices in regenerative medicine, received United States Patent US 10,238,405 on March 26, 2019 entitled “Blood Vessel Treatment […]
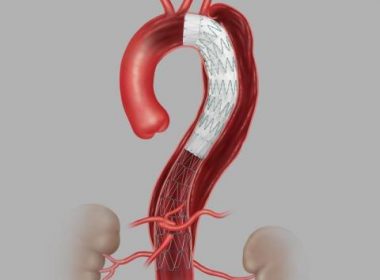
First US Patient Treated with Cook Medical’s Recently Approved Zenith® Dissection Endovascular System
BLOOMINGTON, Ind.–(BUSINESS WIRE)–Following the recent FDA approval of Cook Medical’s Zenith Dissection Endovascular System, Cooper University Health Care in Camden, New Jersey, has treated the first patient in the US with the device as part of Cook’s US commercial launch. “We are committed to helping patients by developing a variety […]
Healogics® Brings Vein Ablation Services to Wound Care Centers®
JACKSONVILLE, Fla., March 21, 2019 /PRNewswire/ — Healogics, the nation’s largest provider of advanced chronic wound care services, today announced they have added vein ablation to their in-house service offerings through a new program called Healogics® VeinCare. This program allows Healogics to provide patients with a higher level of care in a more […]
BD Statement on Paclitaxel-Coated Devices
FRANKLIN LAKES, N.J., March 21, 2019 /PRNewswire/ — BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today issued the following statement related to the recent letter from FDA to health care providers regarding paclitaxel-coated devices: On March 15, the FDA issued a letter to health care providers regarding paclitaxel-coated devices […]
Endonovo Therapeutics Partners with SunMED Medical Systems
LOS ANGELES, CA, March 20, 2019 (GLOBE NEWSWIRE) — Endonovo Therapeutics, Inc. (OTCQB: ENDV) (“Endonovo” or the “Company”), Alan Collier, Endonovo’s Chief Executive Officer, stated “We are pleased to announce our partnership with SunMED Medical Solutions (“SunMED”). SunMED is one of the premier medical equipment distributors and claims experts in the country. […]
Rex Medical Completes Patient Enrollment for the REVEAL Peripheral Atherectomy U.S. Trial
March 19, 2019 10:14 AM Eastern Daylight Time CONSHOHOCKEN, Pa.–(BUSINESS WIRE)–Rex Medical, L.P., a medical device design and development company, today announced the successful completion of enrollment in the Revolution™ Peripheral Atherectomy System for Lower Extremity Peripheral Arterial Revascularization (REVEAL) IDE clinical trial. The REVEAL trial is a single arm, […]
Data from the Non-interventional EMIT-AF/VTE Study Shows Low Thromboembolic and Bleeding Event Rates in Unselected Elderly AF/VTE Patients on Oral, Once-daily LIXIANA®▼ Undergoing Diagnostic or Therapeutic Procedures
MUNICH, March 19, 2019 /PRNewswire/ — In 2019, the Edoxaban Clinical Research Programme will deliver new evidence on LIXIANA®▼ (edoxaban) use in clinical practice. EMIT-AF/VTE is one of the first sets of data to be presented EMIT-AF/VTE is a large observational, multicentre, multinational study on edoxaban peri-procedural management and outcomes It is […]