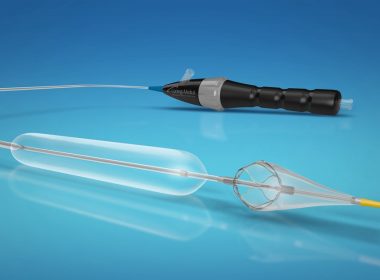
TOKYO, Japan and HORSHAM, U.K., 13 December, 2018 — Otsuka Medical Devices Co., Ltd. (“Otsuka Medical Devices”), a 100% subsidiary of Otsuka Holdings Co., Ltd., and Veryan Medical Ltd. (“Veryan”) announce the completion of the acquisition of Veryan by Otsuka Medical Devices through its UK subsidiary Otsuka Medical Devices UK […]
Peripheral/Endo
Shockwave Announces collaboration With Abiomed on Physician Training
SANTA CLARA, Calif., Dec. 11, 2018 (GLOBE NEWSWIRE) — Shockwave Medical, a pioneer in the development and commercialization of intravascular lithotripsy to treat complex calcified cardiovascular disease, today announced an investment and collaboration agreement with Abiomed, Inc. As outlined by the agreement, Abiomed will invest $15 million in Shockwave and […]
Contego Medical Receives 510(k) Clearance for the Vanguard IEP Peripheral Angioplasty System with Integrated Embolic Protection
RALEIGH, N.C., Dec. 7, 2018 /PRNewswire/ — Contego Medical announced today that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its Vanguard IEP® Peripheral Balloon Angioplasty System with Integrated Embolic Protection. Contego Medical is a medical device company developing and commercializing a suite of next-generation devices that address […]
Inari Medical Announces First Patient Enrolled in FLASH Registry Using the FlowTriever System for Pulmonary Embolism
IRVINE, Calif., Dec. 6, 2018 /PRNewswire/ — Inari Medical, Inc. announced today the enrollment of the first patient in the FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (“FLASH”) using the FlowTriever® System for the treatment of pulmonary embolism (PE). FLASH is a 500-patient prospective, multicenter registry study to evaluate real world outcomes […]
Avinger Announces Commercial Launch in Australia
REDWOOD CITY, Calif., Dec. 06, 2018 (GLOBE NEWSWIRE) — Avinger, Inc. (NASDAQ:AVGR), a commercial-stage medical device company that designs and develops the first-ever image-guided, catheter-based system that diagnoses and treats patients with peripheral artery disease (PAD), today announced the commercial launch of its Pantheris image-guided atherectomy system in Australia. Avinger […]
Caelum Biosciences Announces Two Oral Presentations of Additional Data from Phase 1b Study of Anti-Amyloid mAb CAEL-101 and Preclinical Imaging Study at 60th American Society of Hematology Annual Meeting
NEW YORK, Dec. 04, 2018 (GLOBE NEWSWIRE) — Caelum Biosciences, Inc. (“Caelum”), a company focused on developing treatments for rare and life-threatening diseases, today announced additional global longitudinal strain (“GLS”) data from the Phase 1b study of CAEL-101, a light chain fibril-reactive monoclonal antibody (“mAb”) 11-1F4, in patients with cardiac amyloid light […]
Arizona Patients Successfully Receive the World’s First Bioconvertible IVC Filter Commercially Offered In US
BTG plc (LSE: BTG), the global healthcare company, today announced the first patients outside of a clinical trial have been successfully implanted with the BTG Sentry device – the world’s first bioconvertible IVC filter. The BTG Sentry filter is designed to provide protection from Pulmonary Embolism (PE) for the period […]
Cardinal Health Announces FDA Approval of the INCRAFT® AAA Stent Graft System
DUBLIN, Ohio, November 28, 2018 — Cordis, a Cardinal Health company (NYSE: CAH), today announced that the U.S. Food and Drug Administration (FDA) has approved its INCRAFT® AAA Stent Graft System for use in complex access anatomies. The INCRAFT system is an ultra-low profile and flexible endovascular aneurysm repair (EVAR) system designed […]
MERIT MEDICAL RECEIVES 510(K) APPROVAL FOR EMBOCUBE EMBOLIZATION GELATIN FOR EMBOLIZATION OF HYPERVASCULAR TUMORS
SOUTH JORDAN, Utah – November 29, 2018 – Merit Medical Systems, Inc. (NASDAQ:MMSI), a leading manufacturer and marketer of proprietary disposable devices used primarily in interventional, diagnostic and therapeutic procedures, announces that it has received 510(k) clearance for EmboCube Embolization Gelatin. EmboCube is indicated for use in embolization of hypervascular tumors. The […]
InspireMD Announces Positive Long-Term Safety and Efficacy Data from Ongoing CGuard™ EPS Registries at the Recent 45th Annual Symposium on Vascular and Endovascular Issues, Techniques, Horizons (VEITHsymposium)
TEL AVIV, Israel, Nov. 29, 2018 (GLOBE NEWSWIRE) — InspireMD, Inc. (NYSE American: NSPR), developer of the CGuard™ Embolic Prevention System (EPS) for the prevention of stroke caused by the treatment of carotid artery disease, today announced details from several presentations of updated CGuard™ EPS data at the recent VEITHsymposium […]