(GLOBE NEWSWIRE via COMTEX) –New Data Continue to Reinforce IN.PACT Admiral DCB as a Durable, Frontline Option to Address Treatment Challenges in PAD DUBLIN and LAS VEGAS – November 6, 2018 – Medtronic plc (NYSE:MDT) data presented today reinforce the durability, safety, and effectiveness of the IN.PACT(TM) Admiral(TM) drug-coated balloon […]
Peripheral/Endo
Micro Medical Solutions’ New MicroStent with 120 cm Delivery Catheter Receives CE Mark Approval
WILMINGTON, Mass., Nov. 5, 2018 /PRNewswire/ — Micro Medical Solutions (MMS), an innovator in the field of microvascular interventions designed to improve clinical outcomes and quality of life, announced today that it has received CE Mark approval for a 4Fr.-compatible MicroStent 120 cm delivery catheter, as well as MicroStent devices in five […]
Preclinical Safety Studies Demonstrate That Drug Presence Can Accentuate Pathological Responses at Stent Fracture Sites
LEXINGTON, Mass.–(BUSINESS WIRE)–CBSET, a non-for-profit preclinical research institute dedicated to biomedical research, education and advancement of medical technologies, announced today that its scientists have published data and analyses (“Fracture in drug-eluting stents increases focal intimal hyperplasia in the atherosclerosed rabbit iliac artery”) that “illustrate differences in the dynamic healing responses […]
Acer Therapeutics Submits NDA for EDSIVO™ for the Treatment of vEDS
NEWTON, Mass., Oct. 29, 2018 (GLOBE NEWSWIRE) — Acer Therapeutics Inc. (Nasdaq: ACER), a pharmaceutical company focused on the acquisition, development and commercialization of therapies for serious rare and ultra-rare diseases with critical unmet medical need, today announced that it has submitted a New Drug Application (NDA) to the U.S. […]
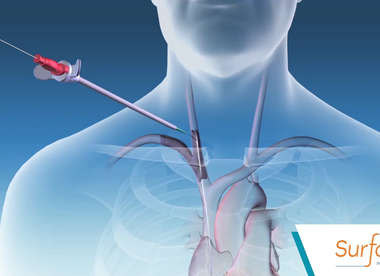
Bluegrass Vascular Announces Positive Results From Independent Study Building On Growing Body Of Evidence For Clinical Application Of The Surfacer System
SAN ANTONIO, Oct. 27, 2018 /PRNewswire/ — Bluegrass Vascular Technologies (Bluegrass Vascular), a private medical technology company focused on innovating lifesaving devices and methods for vascular access procedures, announced today its Surfacer® Inside-Out® Access Catheter System has demonstrated positive commercial use, consistently achieving central venous access in patients with upper body occlusions. The study, […]
Inositec’s INS-3001 Significantly Reduces Vascular Calcification Data Presented at Kidney Week 2018 Indicates
ZURICH–(BUSINESS WIRE)–Inositec, a pioneer in the development of life-saving small molecule drugs based on myo-inositol hexaphosphate (IP6), announced today that INS-3001, a novel vascular calcification inhibitor, demonstrated the ability to significantly inhibit the calcification process in preclinical studies. The build-up of calcium deposits in arterial walls and cardiac valves lead to […]
LeMaitre Vascular Acquires Cardial Business of BD
BURLINGTON, Mass., Oct. 24, 2018 (GLOBE NEWSWIRE) — LeMaitre Vascular, Inc. (Nasdaq:LMAT), announced today that it has acquired the assets of Cardial, a subsidiary of BD (Becton, Dickinson & Company), located in Saint-Étienne, France. The total purchase price for the acquired assets was €2.0 million, structured as €1.1 million of […]
Medtronic Receives FDA Approval for Valiant Navion(TM) Thoracic Stent Graft System
DUBLIN – October 23, 2018 – Medtronic plc (NYSE:MDT) today announced it has received U.S. Food and Drug Administration (FDA) approval for the Valiant Navion(TM) thoracic stent graft system for the minimally invasive repair of all lesions of the descending thoracic aorta, including thoracic aortic aneurysms (TAA), blunt thoracic aortic […]
Aortica™ Corp. Announces Publication of Successful Results Demonstrating Simplification of Fenestrated EVAR Using AortaFit™ Automated Case Planning Software
BELLEVUE, Wash.–(BUSINESS WIRE)–Aortica Corp. today announced the publication of study results in the Journal of Vascular Surgery (JVS) demonstrating the successful use of their investigational AortaFit™ automated case planning software to dramatically simplify the treatment of complex Abdominal Aortic Aneurysm (AAA) disease. The study is an FDA approved physician-sponsored investigational device exemption […]
Avinger Receives CE Marking Approval for Pantheris SV, a New Image-Guided Atherectomy Device Designed to Treat Smaller Vessels
REDWOOD CITY, CA / ACCESSWIRE / October 18, 2018 / Avinger, Inc. (NASDAQ:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), today announced Conformité Européenne (CE) Marking approval of Pantheris SV (Small Vessel), a product line extension of the Lumivascular atherectomy system. CE Marking allows for distribution […]