Audio recording: Learn more about NexGen Medical System and the XCOIL System to remove blood clots from their CEO, Denis Harrington recorded at his office in Wayzata, MN.
Peripheral/Endo
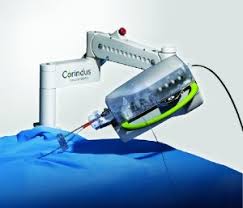
Corindus to Feature the CorPath® GRX System at the Transcatheter Cardiovascular Therapeutics 2018 Conference
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. (NYSE American: CVRS), a leading developer of precision vascular robotics, will feature its CorPath® GRX System at the Transcatheter Cardiovascular Therapeutics (TCT) 2018 Conference, September 21 – 25, in San Diego, CA. Corindus’ CorPath GRX is the first FDA-cleared medical device for robotic-assisted vascular interventions. […]
Hancock Jaffe Laboratories’ VenoValve Featured in Journal of Vascular Surgery Venous and Lymphatic Disorders as a Future Option for Treating Deep Vein Disease
IRVINE, Calif., Sept. 12, 2018 (GLOBE NEWSWIRE) — Hancock Jaffe Laboratories, Inc. (Nasdaq: HJLI, HJLIW), a company specializing in medical devices that restore cardiac and vascular health, announced today that the Journal of Vascular Surgery Venous and Lymphatic Disorders published an article on present and future options for treating deep vein disease, […]
Cook Medical Announces Successful Resolution of 2014 FDA Warning Letter
BLOOMINGTON, Ind.–(BUSINESS WIRE)–Cook Medical today announced that it received a close-out letter from the U.S. Food and Drug Administration resolving a 2014 warning letter for processes related to the quality system at the company’s manufacturing facility in Bloomington, Indiana. The resolution was a direct result of countless hours of hard […]
Vascular Dynamics Announces Initial Enrollment in Rigorously Designed Hypertension Trial
MOUNTAIN VIEW, Calif.–(BUSINESS WIRE)–Vascular Dynamics, Inc. (VDI), a privately held medical device company focused on innovative, minimally invasive device-based solutions for cardiovascular conditions, today announced enrollment of the first patients in an FDA-approved best-in-class pivotal clinical trial. The CALM-2 study is designed to establish safety and efficacy of the novel […]
Prevencio Announces Data Demonstrating HART PAD™ Blood Test Accurately Diagnoses Peripheral Artery Disease in Diabetic Patients
KIRKLAND, Wash., Aug. 27, 2018 /PRNewswire/ — Prevencio, Inc. today announces data demonstrating its HART PAD™test accurately diagnoses peripheral artery disease (PAD) in diabetes mellitus (DM) patients, a patient population in which PAD prevalence has traditionally been difficult to assess. Researchers believe these important findings, presented August 25 at the 2018 European Society of Cardiology […]
Gore Announces Successful Patient Implant of Endovascular Stent Graft for the Ascending Aorta
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) today announced the first implant in conjunction with the Gore ARISE Study of the GORE® Ascending Stent Graft, an investigational device and the only endovascular stent graft specifically designed to treat Type A dissections of the ascending aorta. The successful procedure took place […]
GORE® Molding & Occlusion Balloon for Endovascular Aortic Repair Receives Approval in the United States, Japan, and Europe
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) announced FDA 510(k) clearance, approval from the Japanese Ministry of Health, Labour, and Welfare, and receipt of CE Mark for the innovative GORE® Molding & Occlusion Balloon, a compliant polyurethane balloon catheter designed in close collaboration with clinicians to assist in the expansion […]
Vascular Graft Solutions Reports the First Clinical Use of Its FRAME External Support Technology in Aneurysm Repair of High Flow Arteriovenous Fistulas
TEL AVIV, Israel, Aug. 20, 2018 /PRNewswire/ — Vascular Graft Solutions reports the first clinical use of its FRAME external support technology for aneurysm repair in high flow arteriovenous (AV) fistulas. The first 5 cases were performed by Dr. Vladimir Matoussevitch from Cologne University Hospital in Germany. “Autogenous AV fistula access for hemodialysis is a […]
NuCryo Vascular Announces Commercialization Agreement with Lokai Medical for Innovative Treatment for Peripheral Arterial Disease
SAN JOSE, Calif.–(BUSINESS WIRE)–NuCryo Vascular today announced that the company has signed a commercialization agreement with Lokai Medical, a specialty distributor of coronary/peripheral and interventional devices, to distribute the PolarCath™ Balloon Dilatation System in the United States. Balloon Cryoplasty® has been shown in clinical studies and in everyday procedures to be […]