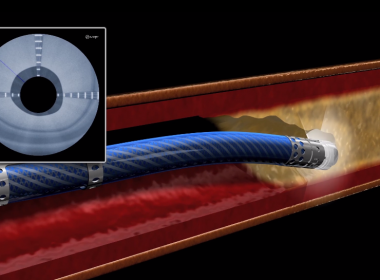
SUNNYVALE, Calif.–(BUSINESS WIRE)–Silk Road Medical, Inc., a company dedicated to preventing the devastating burden of stroke through surgical innovation, today announced the presentation of real-world data for the treatment of patients with carotid artery disease at risk for stroke at the Society for Vascular Surgery 2018 Vascular Annual Meeting (VAM). […]
Peripheral/Endo
PQ Bypass touts 1-year Detour study data
PQ Bypass touted 12-month data today from a trial evaluating its Detour percutaneous femoropopliteal bypass system. Results from the Detour I trial showed “promising 12-month durability” for patients with extremely long blockages in the superficial femoral artery, the company reported. The study included lesions that were longer and more complex than […]
Endologix Reports Positive 1-Year Results from the Ovation® LUCY Study
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (Nasdaq:ELGX), a developer and marketer of innovative treatments for aortic disorders, announced 1-year results from the LUCY (Evaluation of FemaLes who are Underrepresented Candidates for Abdominal Aortic AneurYsm Repair) registry as reported on Saturday, June 23rd at the 2018 Society for Vascular Surgery Annual Meeting. The LUCY study […]
Shape Memory Medical Receives FDA Clearance for the IMPEDE® Embolization Plug
SANTA CLARA, Calif.–(BUSINESS WIRE)–Shape Memory Medical Inc. announced today it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the IMPEDE Embolization Plug. The IMPEDE Embolization Plug is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature. It is available in […]
Avinger Announces Successful Treatment of First Patients with Next-Generation Pantheris in Several Centers throughout the US
REDWOOD CITY, Calif., June 26, 2018 (GLOBE NEWSWIRE) — Avinger, Inc. (Nasdaq:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), today announced that several physicians have successfully treated over 40 patients in the US across 13 sites with the next-generation Pantheris image-guided atherectomy system for the treatment […]
Avinger’s Ocelot Technology Featured in Live Case Transmission at Complex Cardiovascular Catheter Therapeutics (C3) 2018
REDWOOD CITY, Calif., June 21, 2018 (GLOBE NEWSWIRE) — Avinger, Inc. (Nasdaq:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), today announced that its Ocelot image-guided chronic total occlusion (CTO) crossing device was featured in a live case on June 19th at Complex Cardiovascular Catheter Therapeutics (C3) 2018 […]
Mercator MedSystems Receives $11M in Series D Equity Financing Led by Shenzhen Salubris Pharmaceuticals Co.
EMERYVILLE, Calif., June 21, 2018 /PRNewswire/ — Mercator MedSystems, Inc., today announced it has received $11 million in Series D equity financing led by Shenzhen Salubris Pharmaceuticals Co. (Salubris), with participation from current Mercator investors and other undisclosed new investors. The new funding will further advance the clinical development of Mercator’s proprietary micro-infusion catheter systems […]
Saranas Announces Scheduled Presentations at TVT 2018 in Chicago
HOUSTON–(BUSINESS WIRE)–Saranas Inc., a medical device company with innovative technology for real-time detection and monitoring of internal bleeding during endovascular procedures, has been invited to present at the Transcatheter Valve Therapies (TVT) Structural Heart Disease Summit, which will take place June 20-23 at the Sheraton Grand Chicago. Scheduled presentations of […]
Silk Road Medical Announces Key Events for Society for Vascular Surgery 2018 Vascular Annual Meeting
SUNNYVALE, Calif.–(BUSINESS WIRE)–Silk Road Medical, Inc., a company dedicated to preventing the devastating burden of stroke through surgical innovation, today announced key events at the upcoming Society for Vascular Surgery 2018 Vascular Annual Meeting (VAM) June 20-23 at the Hynes Convention Center in Boston. These include real-world data presentations comparing […]
AngioSoma, Inc. Announces Selection of Regulatory Firm for Flagship Drug
HOUSTON, TX, June 18, 2018 (GLOBE NEWSWIRE) — AngioSoma, Inc. (SOAN) is excited to announce that we have identified a regulatory firm who can assist taking our patented flagship drug, Liprostin™, through the FDA approval process. The regulatory firm will begin with a presubmission meeting with the FDA biologic group […]