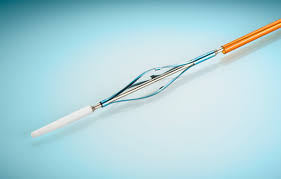
TOKYO, JAPAN – April 24, 2018 – Terumo Aortic announced the European limited market release of the RelayPro Thoracic Stent-Graft System at the 2018 Charing Cross conference. RelayPro is a low profile, next generation device designed to expand the treatment of thoracic endovascular aortic repair (TEVAR) to patients with smaller […]
Peripheral/Endo
Micro Medical Solutions Announces International Scientific Advisory Board to Guide and Advance Clinical Development
WILMINGTON, Mass.–(BUSINESS WIRE)–Micro Medical Solutions (MMS), an innovator in the field of microvascular interventions that improve clinical outcomes and quality of life, announced today that it has completed building a Scientific Advisory Board that includes six international leaders in treatment of peripheral artery disease (PAD) and critical limb ischemia (CLI). […]
Endovascular Aneurysm Sealing (EVAS) with Nellix System Associated with Higher Survival than Traditional Endovascular Aneurysm Repair (EVAR) in New Study
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (Nasdaq: ELGX), a developer and marketer of innovative treatments for aortic disorders, today announced the results of a study, which was presented by Marc Schermerhorn, M.D., Chief of Vascular Surgery at Beth Israel Deaconess Medical Center, on the podium at the Late-Breaking Aortic Trials Session during […]
Shockwave Unveils New Intravascular Lithotripsy Catheter for Calcified Below-the-Knee Peripheral Artery Disease in Europe
Shockwave Medical, a pioneer in the treatment of calcified cardiovascular disease, today announced CE Mark and European commercial availability of the Shockwave S4 Peripheral Intravascular Lithotripsy (IVL) Catheter. Shockwave S4 is a low-profile catheter specifically designed to access and treat challenging calcified lesions in below-the-knee (BTK) arteries frequently associated with […]
Vascutek and Bolton Medical merge as ‘Terumo Aortic’
New company brings together two leaders in the aortic implant market New identity underlines global growth ambitions further to $43m factory investment Merger unites two world-class innovators focused on expanding aortic treatment options Vascutek and Bolton Medical have combined into a single business as it looks to grow its presence […]
Surmodics Announces FDA Clearance of a New .018” Low-Profile PTA Balloon Dilation Catheter
EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–Surmodics, Inc. (NASDAQ: SRDX), a leading provider of medical device and in vitro diagnostic technologies to the healthcare industry, announced it received U.S. Food and Drug Administration (FDA) 510(k) clearance for its .018” Low-Profile percutaneous transluminal angioplasty (PTA) balloon dilation catheter, designed and indicated for a broad […]
Medtronic IN.PACT(TM) Admiral(TM) Drug Coated Balloon Receives FDA Approval to Treat Long SFA Lesions
DUBLIN – April 23, 2018 – Medtronic plc (NYSE: MDT) today announced that it has received U.S. Food and Drug Administration (FDA) approval for the IN.PACT(TM) Admiral(TM) Drug-Coated Balloon (DCB) to treat long superficial femoral artery (SFA) lesions up to 360mm in patients with peripheral artery disease (PAD). Approval was based […]
Intact Vascular Announces $20 Million Series C Financing to Fund Company through PMA Approval of the Tack Endovascular System®
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced that it closed a Series C financing totaling $20 million. This financing is designed to fund the Company through Pre-Market Approval (“PMA”) of the Tack Endovascular System for the treatment of post-angioplasty […]
LimFlow Raises €27 Million ($33.5 Million) in Series C Financing Led by Sofinnova Partners to Advance Innovative Critical Limb Ischemia (CLI) Treatment
PARIS–(BUSINESS WIRE)–LimFlow SA, developer of innovative, peripheral endovascular technology transforming the treatment of critical limb ischemia (CLI), today announced it has secured €27 million ($33.5 million) in an oversubscribed Series C financing. The round was led by Sofinnova Partners, with continued participation from existing round B investors Bpifrance, the French […]
Global Interventional Cardiology and Peripheral Vascular Device Market 2018-2023 – A $26.3 Billion Market Opportunity
DUBLIN, April 10, 2018 /PRNewswire/ — The “Interventional Cardiology and Peripheral Vascular Device Market Report: Trends, Forecast and Competitive Analysis”report has been added to ResearchAndMarkets.com’s offering. The global interventional cardiology and peripheral vascular device market is expected to reach an estimated $26.3 billion by 2023, and it is forecast to grow at a CAGR of 5.5% from […]