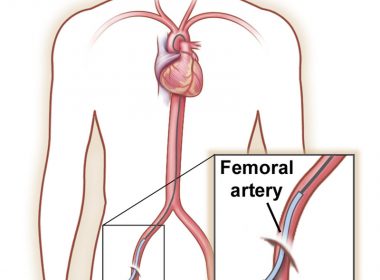
CHICAGO, April 9, 2018 /PRNewswire-USNewswire/ — Has your doctor prescribed an angiogram? If you are not sure what to expect, you’re not alone. The Society for Vascular Surgery angiogram information web page is the number one most-visited of all its patient information pages. What does a vascular surgeon do? In fact, says vascular […]
Peripheral/Endo
The global inferior vena cava (IVC) filters market is forecasted to grow at a CAGR of 11.24% during the period 2018-2022
NEW YORK, April 3, 2018 /PRNewswire/ — About Inferior Vena Cava (IVC) Filters The IVCF is a type of vascular filter that is inserted into the inferior vena cava to prevent blood clots from moving from the veins in the legs and pelvis to the lung, which can lead to life-threatening pulmonary embolism […]
IVC Filter Lawsuit News: C.R. Bard Ordered to Pay $3.6 Million at Conclusion of First Federal Bellwether Trial, Bernstein Liebhard LLP Reports
NEW YORK, April 2, 2018 /PRNewswire/ — C.R. Bard, Inc. has been ordered to pay $3.6 million to a Georgia woman who suffered serious complications after the company’s G2 inferior vena cava (IVC) filter fractured inside her body. The case was the first to go to trial in the U.S. District Court, District of Arizona, where more than […]
Symic Bio Announces 12-Month Results from the SHIELD Trial of SB-030 in Peripheral Vascular Disease
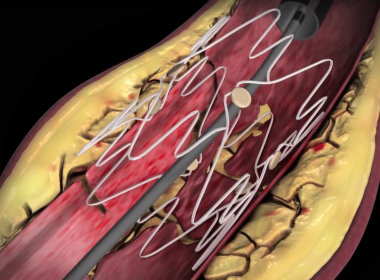
SAN FRANCISCO, April 2, 2018 /PRNewswire/ — Symic Bio, a biopharmaceutical company developing novel matrix-targeting biotherapeutics, today announced 12-month results from the Phase 1/2 SHIELD trial evaluating SB-030 in patients with peripheral vascular disease undergoing angioplasty. Under an extended trial protocol allowing assessment of secondary endpoint measurements at 12 months following intervention, patients […]
MIVI Neuroscience Begins European Commercialization After Achieving CE Mark Approval Of The R4Q Revascularization Catheter For Ischemic Stroke
EDEN PRAIRIE, Minn., March 29, 2018 /PRNewswire/ — MIVI Neuroscience, Inc. announced today it has received CE Mark approval for the R4Q Revascularization Catheter for the removal of fresh, soft emboli and thrombi in the peripheral and neurovascular systems. The CE Mark approval is a significant accomplishment for MIVI Neuroscience. This approval enables the […]
Inari Medical Announces Completion of $27 Million Series C Financing
IRVINE, Calif., March 29, 2018 /PRNewswire/ — Inari Medical, Inc., announced today the close of a Series C financing totaling $27.0 million. The financing was led by new investor Gilde Healthcare and was joined by all of Inari’s existing investors including Versant Ventures and U.S. Venture Partners. Geoff Pardo of Gilde Healthcare will join Inari’s Board […]
IDx Founder Awarded Patent for System that Automatically Detects Measure of Cardiovascular Health
IOWA CITY, Iowa, March 29, 2018 /PRNewswire/ — IDx, a privately-held AI diagnostics company, today announced its founder and president, Michael Abramoff, MD, PhD, has been awarded U.S. Patent No. 9,924,867 for a system that can automatically measure arterial and venous diameters in blood vessels, an important predictor of cardiovascular health. “It is exciting that […]
Intact Vascular Welcomes TOBA BTK Trial One-Year Clinical Study Results Publication in Catheterization and Cardiovascular Intervention
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today welcomed the publication of the Tack Optimized Balloon Angioplasty Below the Knee (TOBA BTK) clinical trial results in the current issue of Catheterization and Cardiovascular Intervention. This multi-center pilot study focused on collecting data supporting the safety and performance of the […]
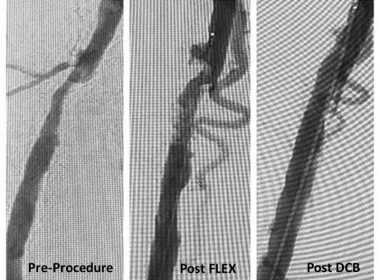
VentureMed Group’s FLEX Scoring Catheter® was Featured in a Successful Live Case at ISET 2018
TOLEDO, Ohio, March 26, 2018 /PRNewswire/ — The FLEX Catheter by VentureMed Group, an innovator in vessel prep, was featured in a live case transmission at the International Symposium of Endovascular Therapy (ISET), in Hollywood, FL. ISET focuses on the advancement of endovascular education, promoting discussions, and providing opportunities to explore new technologies and innovative […]
Pythagoras Medical Receives CE-Mark for the ConfidenHT™ System
HERTSLIYA, Israel, March 26, 2018 /PRNewswire/ –Pythagoras Medical, a cutting edge medical device company established by Rainbow Medical, Israel’s premier medical device investment group, announced today that its ConfidenHT(TM) System has received a European CE Mark. ConfidenHT(TM) is a novel system for patients with resistant hypertension, which improves the efficacy […]