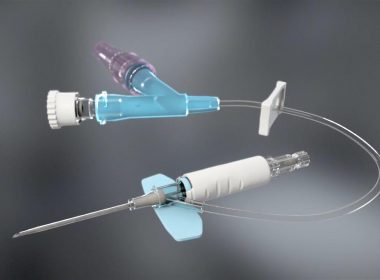
MINNEAPOLIS–(BUSINESS WIRE)– Smiths Medical, Inc. today announced the U.S. commercial launch of Delta Med SpA’s DeltaVen® Closed System Catheter and the Food and Drug Administration (FDA) clearance. DeltaVen® is the next advancement in Peripheral IV Catheters (PIVC); offering the needle, extension tubing, and an option for a needleless connecter in an “all in one” […]
Peripheral/Endo
Access Scientific, LLC Announces Discovery of the Cause of POWERWAND™ Catheters’ ZERO Bloodstream Infections in over 35,000 Catheter-Days of Published Use
SAN DIEGO–(BUSINESS WIRE)–Unprecedented. That’s what Access Scientific is calling the dramatic results from a leading biofilm laboratory that explain why the company’s POWERWAND™ midlines and extended-dwell catheters consistently report zero associated bloodstream infections (BSIs). Independent, peer-reviewed published studies and scientific posters on the company’s POWERWAND™ catheters – which now tally […]
Endologix Reports Positive Results from Global ENCORE Analysis with Polymer Endovascular Aneurysm Repair (EVAR) Using Ovation Abdominal Stent Graft Systems
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (Nasdaq:ELGX), a developer and marketer of innovative treatments for aortic disorders, today announced the first results from ENCORE, a pooled, global analysis of several prospective clinical trials and registries studying polymer endovascular aneurysm repair (Polymer EVAR) using Ovation® Abdominal Stent Graft Systems. ENCORE is a pooled, […]
Cook Medical Wins Quick Judgment in Second IVC Filter Litigation Case, Commits to Continued Fight for Life-Saving Technology
BLOOMINGTON, Ind.–(BUSINESS WIRE)–District Court Judge Richard L. Young entered judgment in favor of Cook Medical in the second bellwether case on IVC filters. This ruling comes just months after Cook Medical won a favorable verdict in the first bellwether IVC filter litigation case with a unanimous decision from the Evansville, […]
United States Patent and Trademark Office Issues Multiple Patents on New Arterial & Venous Embolic Device to Control Bleeding, Seal Leaks, and De-Vascularize Tumors
PARK CITY, Utah–(BUSINESS WIRE)–The United States Patent & Trademark Office (USPTO) issued two new patents today for the GPX Embolic Particle device, a biocompatible catheter delivered embolic technology designed for use in arteries and veins. A scientific presentation of this vascular embolic device titled GPX: A New Proprietary In Situ […]
Endospan Appoints Kevin J. Mayberry as CEO
HERZLIA, Israel–(BUSINESS WIRE)–Endospan, a pioneer in off-the-shelf endovascular repair of Aortic Arch Disease including aneurysms and dissections, today announced that it has appointed Kevin J. Mayberry as Chief Executive Officer (CEO), effective immediately. He replaces Alon Shalev, who will continue as a director of Endospan. Previously, Mayberry served in various R&D and […]
Reflow Medical announces enrollment of its first patients in the Wing-IT IDE clinical trial
San Clemente, Calif., March 12, 2018 /PRNewswire/ — Reflow Medical Inc., a developer of innovative medical devices to combat cardiovascular disease, announced that the first patients have been enrolled in a prospective, multi-center, non-randomized study intended to evaluate the ability of the Reflow Wingman Catheters to cross chronic total occlusions […]
XableCath Granted United States Patent for its Peripheral Arterial Catheters
SALT LAKE CITY–(BUSINESS WIRE)– XableCath, Inc., innovators of products designed to aid in the treatment of peripheral artery disease (PAD), announced that it has been granted a second U.S. Patent, No. 9,826,995, covering its XableCath™ catheter. The patent specifically describes a catheter that controls tissue contact and has a rigid […]
Cardiovascular Systems Presents LIBERTY 360° 18-Month Outcomes at CRT18 Interventional Cardiology Conference
ST. PAUL, Minn.–(BUSINESS WIRE)–Cardiovascular Systems, Inc. (CSI®) (NASDAQ: CSII) a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, presented 18-month outcomes from its LIBERTY 360° study at the Cardiovascular Research Technologies (CRT) 2018 interventional cardiology conference in Washington, D.C. The […]
Endologix, Inc. Announces Enrollment of the First Patient in EVAS2 IDE Clinical Study of the Nellix® EndoVascular Aneurysm Sealing System
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (Nasdaq:ELGX), a developer and marketer of innovative treatments for aortic disorders, announced today that the first patient was treated in the EVAS2 IDE Confirmatory Clinical Study of the investigational Nellix® EndoVascular Aneurysm Sealing (EVAS) System by Sajjad M. Hussain, M.D., Chief of the Department of Vascular Surgery […]