IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (Nasdaq:ELGX), a developer and marketer of innovative treatments for aortic disorders, announced today that the first patient was treated in the EVAS2 IDE Confirmatory Clinical Study of the investigational Nellix® EndoVascular Aneurysm Sealing (EVAS) System by Sajjad M. Hussain, M.D., Chief of the Department of Vascular Surgery […]
Peripheral/Endo
Symic Bio Announces Results of Locally-Administered Therapeutic SB-030 in Preclinical Model of Vascular Intervention
SAN FRANCISCO, March 5, 2018 /PRNewswire/ — Symic Bio, a biopharmaceutical company developing novel extracellular matrix targeting drugs, today announced results from a preclinical study of locally-applied therapeutic SB-030 in the reduction of inflammatory response and clot formation. In a porcine arteriovenous shunt model for vascular procedures such as bypass grafts and angioplasty, […]
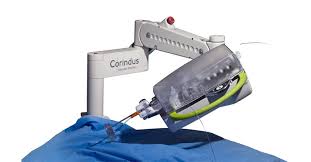
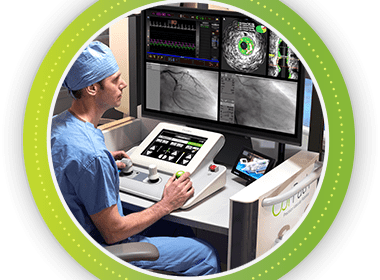
Corindus Receives FDA Clearance for First Automated Robotic Movement in technIQ™ Series for CorPath GRX Platform
WALTHAM, Mass.–(BUSINESS WIRE)– Corindus Vascular Robotics Inc. (NYSE American: CVRS), a leading developer of precision vascular robotics, announced today that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the first automated robotic movement designed for the CorPath GRX platform. The proprietary software feature, named “Rotate on Retract” […]
New Biocompatible Embolic Device to Control Blood Flow & Treat Tumors Presented at the Leipzig Interventional Congress (LINC)
GPX is a biocompatible gel-particle embolic device that transforms from low-viscosity particles in a syringe to a solid particle after injection into a vessel LEIPZIG, GERMANY, February 01, 2018 /24-7PressRelease/ — Two scientific abstracts of a new vascular embolic device were presented this week at the 2018 Leipzig Interventional Congress […]
Abbott and Surmodics Announce Agreement for Next-Generation Drug-Coated Balloon
ABBOTT PARK, Ill. and EDEN PRAIRIE, Minn., Feb. 27, 2018 /PRNewswire/ — Abbott (NYSE: ABT) and Surmodics (NASDAQ: SRDX) today announced that the companies have entered into an agreement whereby Abbott will have exclusive worldwide commercialization rights for Surmodics’ SurVeil® drug-coated balloon to treat the superficial femoral artery, which is currently being evaluated in a U.S. pivotal […]
John McDermott Steps Down as Chief Executive Officer of Endologix, Inc.
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (Nasdaq:ELGX), a developer and marketer of innovative treatments for aortic disorders, announced today that John McDermott is stepping down as the Company’s Chief Executive Officer. To facilitate a smooth transition, Mr. McDermott has committed to remaining in his current role until a successor is found. Daniel T. […]
Delray Beach Medical Center Performs First Robotic-Assisted Peripheral Vascular Intervention Using Corindus CorPath® GRX System Following FDA Clearance
WALTHAM, Mass.–(BUSINESS WIRE)– Corindus Vascular Robotics (Formerly known as Corindus), Inc. (NYSE American: CVRS), a leading developer of precision vascular robotics, announced today the successful completion of the first robotic-assisted peripheral vascular intervention procedure using the recently FDA-cleared CorPath GRX System. The procedure was performed by Joseph Ricotta, M.D., Medical Director […]
PQ Bypass Announces First Patient Treated in Landmark DETOUR II Trial Evaluating New Treatment Approach for Clogged Leg Arteries
SUNNYVALE, Calif.–(BUSINESS WIRE)– PQ Bypass, a clinical-stage medical technology company that has developed the DETOUR procedure, a novel treatment for long (>15 cm) superficial femoral artery (SFA) blockages, announced enrollment of the first patients in the pivotal DETOUR II Trial in the United States. The trial is a prospective, single-arm clinical […]
Corindus Receives FDA Clearance for CorPath® GRX System in Peripheral Vascular Interventions
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. (NYSE American:CVRS), a leading developer of precision vascular robotics, announced today that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use of its CorPath GRX System in peripheral vascular interventions. The CorPath System is the first and only FDA-cleared […]
Cook Medical Realigns One-Third of Its Global Workforce to Better Support Customers, Enable Future Growth and Provide Opportunity for Employees
BLOOMINGTON, Ind.–(BUSINESS WIRE)– Today, Cook Medical announced key changes that simplify its organizational structure to better support customers. These changes realign the current sales, marketing, research and development, and customer service teams in addition to establishing new distribution channel management and medical education teams. The changes are a direct result of feedback […]