FRANKLIN LAKES, N.J., Jan. 18, 2018 /PRNewswire/ — Becton, Dickinson and Company (NYSE: BDX), a leading global medical technology company, today announced the completion of enrollment in the Lutonix below-the-knee (BTK) trial and plans to submit a pre-market approval application in late 2018. The Lutonix BTK trial is a prospective, multicenter, randomized, […]
Peripheral/Endo
Secant Group Develops the First Synthetic Regenerative Cardiovascular Graft
TELFORD, Pa.–(BUSINESS WIRE)–Secant Group, in partnership with its sister company SanaVita Medical, announce game changing technology to advance cardiovascular regenerative medicine with the development of a synthetic, small bore, vessel that encourages endogenous regeneration and new vessel formation. The technology is based on the company’s sophisticated textile forming capabilities that […]
Global Peripheral Vascular Devices Market Analysis 2017 and Forecasts 2018-2022 by Type – A Potential $12.6 Billion Market – Research and Markets
DUBLIN–(BUSINESS WIRE)–The “Peripheral Vascular Devices Market by Type (Angioplasty Balloon, Stent, Catheters, Plaque Modification, Hemodynamic Flow Alteration, IVC Filters, Guidewires) – Global Forecast to 2022” report has been added to Research and Markets’ offering. The global peripheral vascular devices market is expected to reach USD 12.63 Billion by 2022 from USD 9.09 Billion in […]
Teleflex Surpasses 200,000 Successfully Reprocessed ClosureFast® Catheters
WAYNE, Pa.–(BUSINESS WIRE)– Teleflex Incorporated (NYSE: TFX), a leading global provider of medical technologies for critical care and surgery, today announced that more than 200,000 ClosureFast® Catheters have been successfully reprocessed by the company’s partner, Northeast Scientific, Inc. (NES), since NES received FDA clearance for reprocessing the popular vein ablation catheter in 2011. […]
Centerline Biomedical Completes Fifth Preclinical Study
CLEVELAND, Jan. 4, 2018 /PRNewswire/ — As Centerline Biomedical, Inc. prepares to meet with investors, strategic partners and companies in the healthcare industry during the JP Morgan Healthcare Conference, it successfully completed a fifth preclinical study at Cleveland Clinic facilities evaluating its surgical navigation system, IOPS, the Intra-Operative Positioning System. This study […]
Peripheral Vascular Devices Market Worth 12.63 Billion USD by 2022
PUNE, India, January 4, 2018 /PRNewswire/ — According to a new market research “Peripheral Vascular Devices Market by Type (Angioplasty Balloon, Stent, Catheters (Angiography, IVUS), Plaque Modification (Atherectomy, Thrombectomy), Hemodynamic Flow Alteration (Embolic Protection), IVC Filters, Guidewires) – Global Forecast to 2022“, published by MarketsandMarkets™, the market is expected to reach USD 12.63 Billion by 2022 from USD 9.09 […]
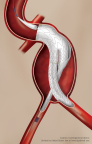
First Patient Enrolled in Investigational Study of the GORE EXCLUDER Conformable AAA Endoprosthesis with ACTIVE CONTROL System
FLAGSTAFF, Ariz.–(BUSINESS WIRE)– W. L. Gore & Associates, Inc.(Gore) today announced the first implant of the GORE® EXCLUDER® Conformable AAA Endoprosthesis in the United States. The successful procedure took place on December 19, 2017 at Maimonides Medical Center in New York by Robert Rhee, MD, Chief of Vascular and Endovascular Surgery, and National […]
Avinger Announces Treatment of First Patients Globally With Next Generation Pantheris
REDWOOD CITY, Calif., Jan. 03, 2018 (GLOBE NEWSWIRE) — Avinger(NASDAQ:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), announced that Arne Schwindt, M.D., a vascular surgeon at St. Franziskus Hospital in Münster, Germany, has successfully treated the first seven patients with the next generation Pantheris Lumivascular atherectomy system. […]
Avinger Announces 510(k) Filing of Next Generation Pantheris Device
REDWOOD CITY, Calif., Dec. 21, 2017 (GLOBE NEWSWIRE) — Avinger, Inc. (NASDAQ:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), announced the Company submitted a new 510(k) application to the U.S. Food & Drug Administration (FDA) for its next generation Pantheris® Lumivascular atherectomy system, the first-ever image-guided […]
Mentice Acquires Endovascular Business from Medical Simulation Corporation
GOTHENBURG, Sweden–(BUSINESS WIRE)–Mentice boosts its US development presence and adds a clinical training service line with the acquisition of Medical Simulation Corporation’s (MSC) Medical Products division. Mentice is the global leader of simulation-based performance solutions for endovascular therapies. Under the agreement, Mentice will acquire the assets of the MSC’s Medical […]