REDWOOD CITY, Calif., Dec. 20, 2017 (GLOBE NEWSWIRE) — Avinger, Inc. (NASDAQ:AVGR), a leading developer of innovative treatments for peripheral artery disease (PAD), today announced Conformité Européenne (CE) Marking approval for its next generation Pantheris Lumivascular atherectomy system, the first-ever image-guided atherectomy device for the treatment of PAD. The novel […]
Peripheral/Endo
XableCath Scores FDA Clearance for Broadly Effective Catheter
SALT LAKE CITY–(BUSINESS WIRE)– XableCath, a clinical stage medical device company, announced its XableCath blunt tip support catheter has received clearance from the FDA. The blunt tip catheter facilitates true lumen passage of lesions, both above and below the knee, in the peripheral vasculature. Procedures using the XableCath blunt tip catheters can […]
Centers Commence Enrollment in KNOCOUT PE Study to Measure the Impact of Shorter, Even Safer EKOS® Therapy Protocols for Pulmonary Embolism
BOTHELL, Wash.–(BUSINESS WIRE)–BTG plc (LSE: BTG), the global specialist healthcare company, today highlights the commencement of the KNOCOUT PE study. The KNOCOUT PE study will measure how hospitals and patients are benefitting from a new standard of care in the treatment of Pulmonary Embolism utilizing EKOS® therapy with faster, and even […]
Saranas Named Finalist for Prestigious International Cardiovascular Innovation Award
HOUSTON–(BUSINESS WIRE)–Saranas, a medical device company with a new technology for real-time detection and monitoring of internal bleeding during endovascular procedures, today announced the company was named as one of the Top 4 Innovators at the International Conference for Innovations in Cardiovascular Systems (ICI) in Tel Aviv, Israel. The ICI […]
Velano Vascular Named to 2017 FierceMedTech’s “Fierce 15”
SAN FRANCISCO, Dec. 12, 2017 (GLOBE NEWSWIRE) — Velano Vasculartoday announced that it was named by FierceMedTech as one of 2017’s Fierce 15 med tech companies, acknowledging the company as one of the most promising private companies in the industry for its breakthrough work addressing the world’s growing vascular access challenges. More than one […]
PQ Bypass Receives IDE Approval to Initiate Study of First-of-its-Kind Procedure for Patients Suffering From Peripheral Artery Disease
SUNNYVALE, Calif.–(BUSINESS WIRE)–PQ Bypass, Inc., today announced it has received conditional approval of its investigational device exemption (IDE) from the U.S. Food and Drug Administration (FDA) to initiate the pivotal DETOUR II clinical trial. As the first-ever pivotal trial for percutaneous femoropopliteal bypass, DETOUR II will evaluate the safety and […]
Janssen Submits Supplemental NDA to FDA Seeking New Indications for XARELTO (rivaroxaban) for Patients With Chronic Coronary and/or Peripheral Artery Disease (CAD/PAD)
RARITAN, N.J., Dec. 11, 2017 /PRNewswire/ — Janssen Research & Development today announced it has submitted a supplemental New Drug Application (sNDA) to the U.S. Food and Drug Administration (FDA) for two new XARELTO® (rivaroxaban) vascular indications: reducing the risk of major cardiovascular (CV) events such as CV death, heart attack or stroke […]
PVD Patients Boomeranging at Higher Rates After Intervention, Study Shows
By Ken Dropiewski, Prime-Core Executive Search (ken@prime-core.com) As we progress to value-based healthcare system, it’s more important than ever to keep readmissions to a minimum. A recent study, though, found that more than 17 percent of patients undergoing peripheral revascularization for PVD were readmitted within 30 days of having the procedure.The unplanned […]
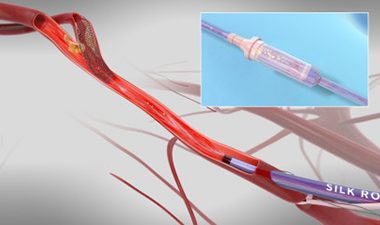
Updated TCAR Data From Silk Road Medical’s ROADSTER 1 and 2 Studies Presented at VEITHsymposium
SUNNYVALE, Calif., Nov. 29, 2017 /PRNewswire/ — Silk Road Medical, a company dedicated to preventing the devastating burden of stroke through surgical innovation, announced multiple presentations at the 45th annual VEITHsymposium highlighting the safety and efficacy of the company’s ENROUTE® Transcarotid Neuroprotection and Stent System, the first and only products specifically designed and […]
Vascular Flow Technologies Forges Strategic Partnership With Biovic to Develop the New Avatar SLF Vascular Graft
DUNDEE, Scotland, November 21, 2017 /PRNewswire/ — Vascular Flow Technologies, the medical device company who developed the proprietary Spiral Laminar Flow (SLF(TM)) technology to re-establish natural blood flow for enhanced patient outcomes, today announces the successful conclusion of a strategic intellectual property out-licencing agreement with Biovic Sdn bhd. Biovic are […]