by Ken Dropiewski, Prime-Core Executive Search (ken@prime-core.com) Balloon expandable stents have been the mainstay in treating blockages in the iliac arteries for years. However, recent data suggests a change is in order. According to researchers who recently compared the two versions in a large group of patients being treated in […]
Peripheral/Endo
Medtronic Plans for Renal Denervation Pivotal Trial After New Study Shows Significant Blood Pressure Reductions
Published Simultaneously in The Lancet, the Late-Breaking SPYRAL HTN-OFF MED Study at ESC Successfully Isolates RDN Treatment Effect to Show Compelling Efficacy and Safety of Hypertension Procedure DUBLIN and BARCELONA – August 28, 2017 – Medtronic plc (NYSE:MDT) today announced its intent to move forward with a new renal denervation pivotal trial […]
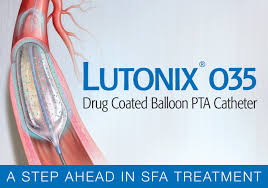
C. R. Bard, Inc. (BCR) Snags FDA Premarket Approval of The Lutonix 035 Drug Coated Balloon As The First And Only DCB
C. R. Bard Receives Fda Premarket Approval Of The Lutonix® 035 Drug Coated Balloon As The First And Only Dcb For The Treatment Of Patients With Dysfunctional Av Fistulae New Option to Preserve Vascular Access and Help Hemodialysis Patients Extend Time Between Reinterventions MURRAY HILL, N.J.–(BUSINESS WIRE)–C. R. Bard, Inc. […]
Ra Medical Looks for Potential IPO in Early 2018
Ra Medical finally received FDA approval this year after three years, now they will be shooting for an IPO. Ra Medical CEO Irwin aims for Feb 2018 IPO AUGUST 24, 2017 BY FINK DENSFORD, MassDevice In May, Ra Medical received a nod from the FDA for its Dabra arteriosclerosis laser, clearing it for use in the […]
Malin Announces U.S. FDA Approval for New Hourglass™ Peripheral Embolisation Plug
DUBLIN–(BUSINESS WIRE)–Malin Corporation plc. (ISE:MLC, “Malin”), an Irish based and globally operating life sciences company, today announced that its EMBA device, known as the Hourglass™ Peripheral Embolisation Plug, was granted U.S. FDA 510(k) clearance to commence marketing in the US. The Hourglass™ peripheral embolisation plug represents a breakthrough in peripheral […]
Getinge Announces Full U.S. Availability Of Pulsar-18 Self-Expanding Stent From BIOTRONIK For Patients With Peripheral Artery Disease
WAYNE, N.J., Aug. 22, 2017 /PRNewswire/ — Getinge, a leading global provider of innovative medical technology, today announces the full U.S. market release of the Pulsar®-18 stent from BIOTRONIK. Pulsar-18 is the only available self-expanding stent for blocked superficial femoral arteries with a 4-French (4F) delivery system. Getinge currently distributes BIOTRONIK’s portfolio of […]
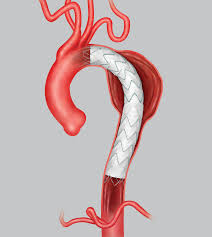
Endologix Announces Positive Clinical Results From The LEOPARD Clinical Study
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (NASDAQ:ELGX), a developer and marketer of innovative treatments for aortic disorders, today announced positive interim results from the LEOPARD (Looking at Evar Outcomes by Primary Analysis of Randomized Data) clinical study. LEOPARD is the first and only head-to-head, prospective, multi-center, randomized clinical study comparing currently available endovascular abdominal aortic stent grafts. LEOPARD […]
Cook has Class I Recall for Zenith Alpha Thoracic Graft
Cook said to be recalling this device because of blood clots that can form within the graft after implantation. Zenith Alpha Thoracic Endovascular Graft by Cook Medical: Class I Recall – Potential Formation of Thrombus Inside Device AUDIENCE: Risk Manager, Cardiology, Surgery, Patient ISSUE: Cook Medical Inc. is recalling the […]
VentureMed Group Raises $15 Million Series B Equity Financing
TOLEDO, Ohio, Aug. 15, 2017 /PRNewswire/ — VentureMed Group®, Inc., (“VMG” or the “Company”) a medical device company developing and commercializing next-generation endovascular products to treat patients suffering from peripheral artery disease (PAD), today announced it had raised $15 million in new equity financing. The Series B Round was led by new investor Endeavour […]
Philips completes acquisition of The Spectranetics Corporation
Amsterdam, the Netherlands and Colorado Springs, CO, U.S. – Royal Philips (NYSE: PHG; AEX: PHIA), a global leader in health technology, today announced that it has completed the acquisition of The Spectranetics Corporation (NASDAQ: SPNC), a U.S.-based global leader in vascular intervention and lead management solutions. Spectranetics’ financial results will be consolidated as part of […]