FREMONT, Calif.–(BUSINESS WIRE)–Shockwave Medical, a pioneer in the treatment of calcified cardiovascular disease, today announced two milestones for its Lithoplasty® System for the treatment of calcified plaque in patients with peripheral artery disease (PAD): enrollment of the first patient in the global DISRUPT PAD III clinical trial at the Medical […]
Peripheral/Endo
TVA Medical Release: Study Published In Prominent Journal Supports Use Of Innovative Endovascular Hemodialysis Access Technology
AUSTIN, Texas, June 15, 2017/PRNewswire/ — TVA Medical, Inc. today announced the online publication of positive results from the Novel Endovascular Access Trial (NEAT) evaluating its everlinQTM endoAVF System. The 12-month data published online in the American Journal of Kidney Diseases (AJKD), the official journal of the National Kidney Foundation, […]
San Francisco Surgeon Dr. Timothy Chuter Receives the 2017 Jacobson Innovation Award of the American College of Surgeons
Newswise — CHICAGO : The 2017 Jacobson Innovation Award of the American College of Surgeons (ACS) was presented to Timothy A.M. Chuter, BM BS, DM, FACS, at a dinner held in his honor in Chicago, Ill. Dr. Chuter is a professor of surgery at the University of California, San Francisco […]
Distinguished Vascular Surgeon, Douglas Paget, MD, FACS, RVT will be Spotlighted in The Leading Physicians of the World
PR NewsChannel: The International Association of HealthCare Professionals is pleased to welcome Douglas Paget, MD, FACS, RVT, Vascular Surgeon to their prestigious organization with his upcoming publication in The Leading Physicians of the World. Dr. Paget is a highly trained and qualified surgeon with an extensive expertise in all facets […]
Europe’s LimFlow Hires a COO and Opens Bay Area Office
LimFlow Expands Senior Management Team And Opens Silicon Valley Office PARIS–(BUSINESS WIRE)–LimFlow SA, developer of minimally-invasive technology for the treatment of end-stage critical limb ischemia (CLI), a severe form of peripheral artery disease (PAD), today announced the expansion of the company’s senior management team. Sophie Humbert, PhD, has been appointed […]
Cagent Vascular Completes Enrollment of PRELUDE Study Using Serranator Device
WAYNE, Pa.–(BUSINESS WIRE)–Cagent Vascular, a developer of next generation technology for vessel dilatation in cardiovascular disease interventions, including the FDA 510(k) cleared Serranator® Alto PTA Serration Balloon Catheter, announces the completion of enrollment in the First-in-Human PRELUDE Study. The purpose of this prospective, single-arm, multicenter feasibility study is to show […]
Avera Names Myles Greenberg, MD, as CEO of Alucent Medical
SIOUX FALLS, S.D.–(BUSINESS WIRE)–Myles D. Greenberg, MD, was named President and CEO of Alucent Medical, Inc., a new company licensed to develop a novel drug/device combination therapy for the treatment of peripheral vascular disease (PVD). The treatment, Natural Vascular Scaffolding (NVS)TM, has recently gained FDA approval to move forward with […]
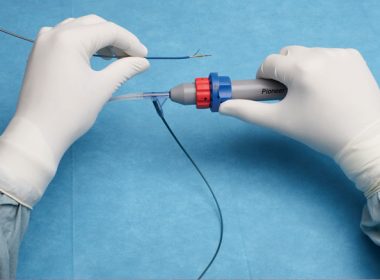
Philips announces the relaunch of its Pioneer Plus catheter, the only re-entry device with intravascular ultrasound guidance
Phillips News Release AMSTERDAM, the Netherlands – Royal Philips (NYSE: PHG AEX: PHIA) today announced the relaunch of its innovative Pioneer Plus catheter, the first and only re-entry device with intravascular ultrasound (IVUS) capabilities and needle deployment designed to assist arterial vessel intervention. IVUS captures images of vessels in the […]
RA Medical’ DABRA Begins First Commercial, FDA-Cleared, In-Patient Use
NEW ORLEANS, La.–(BUSINESS WIRE)–Ra Medical Systems, makers of cardiovascular and dermatology catheters and excimer lasers, today announced the commercial launch in the United States of the Company’s groundbreaking DABRA™ System for the treatment of Peripheral Artery Disease. This follows FDA clearance earlier this week. Dr. Athar Ansari of the California […]
First-in-Man study of novel sirolimus-coated balloon completes enrolment
PCRonline/News/Industry Press Releases Med Alliance has announced completion on schedule of patient enrolment in the First-in-Man (FIM) study of SELUTION™, a novel sirolimus-coated balloon. 50 patients have been enrolled between 26 October 2016 and 23 May 2017 across four German centres: Franziskus Krankenhaus, Berlin; Evangelisches Krankenhaus Hubertus, Berlin; Vivantes Klinikum […]