Nov 19, 2024 Definitive One Year Data from PMA Application to be Presented Wednesday at 51st Annual VEITH Symposium Company to Host a Conference Call with the PIs Presenting the Pivotal Trial Data on Wednesday, November 20th at 2:00 PM Eastern Time – Click Here to Access IRVINE, CA / November 19, […]
Peripheral/Endo
Dr. Pedro Martinez-Clark, Founder of Amavita Heart and Vascular™, Partners with LimFlow®, an FDA Approved System for Chronic Limb-Threatening Ischemia Patients
MIAMI, Nov. 7, 2024 /PRNewswire/ — Pedro Martinez-Clark, MD, FACC, a renowned Harvard-trained cardiologist and founder of Amavita Heart and Vascular™, has joined forces with LimFlow® to offer an innovative solution for patients suffering from chronic limb-threatening ischemia (CLTI)….
Final Rounds of Late-Breaking Clinical Trial Results Announced at VIVA24
LAS VEGAS, Nov. 5, 2024 /PRNewswire/ — The VIVA Foundation, a not-for-profit organization dedicated to advancing the field of vascular medicine and intervention through education and research, announces the results for the final two rounds of Late-Breaking Clinical Trials presented at…
Endologix Announces 36-Month Results of DETOUR2 Study at 2024 VIVA Late-Breaking Clinical Trial Session
IRVINE, Calif.–(BUSINESS WIRE)–Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, today announced the final 36-month results of the DETOUR2 Study. The DETOUR System offers a unique approach to treating complex peripheral arterial disease (PAD), enabling physicians to […]
New Late Breaking Data Show Patients Treated with Penumbra’s Computer Assisted Vacuum Thrombectomy Technology for Pulmonary Embolism Experience Shorter Hospital Stays and Fewer Complications Compared to Other Treatment Options
Data presented for the first time this week at Vascular Interventional Advances (VIVA) 2024 Conference Findings reinforce the clinical, health economic and cost benefits of computer assisted vacuum thrombectomy to patients and the overall health system ALAMEDA, Calif., Nov. 5, 2024…
First Rounds of Late-Breaking Clinical Trial Results Announced at VIVA24
LAS VEGAS, Nov. 4, 2024 /PRNewswire/ — The VIVA Foundation, a not-for-profit organization dedicated to advancing the field of vascular medicine and intervention through education and research, announces the results for the first two rounds of Late-Breaking Clinical Trials presented at…
Medtronic: New data adds to body of research demonstrating safety and effectiveness of atherectomy
Comparative data and systematic literature review released at VIVA 2024 show breadth and depth of data supporting atherectomy as a treatment for peripheral arterial disease Medtronic, the global leader in healthcare technology, today announced results from two studies evaluating the utility of atherectomy for peripheral endovascular interventions. Results from both […]
Shockwave Medical Unveils First Clinical Outcomes of New IVL Platform in Late-Breaking Presentation at VIVA 2024
Novel Intravascular Lithotripsy (IVL) catheter demonstrates ability to modify calcium safely, enabling treatment and crossing of tightly-stenosed lower limb lesions Technology has received U.S. FDA clearance for patients with peripheral arterial disease LAS VEGAS, Nov. 4, 2024…
AKURA MEDICAL SECURES FDA APPROVAL FOR US PIVOTAL TRIAL IN PULMONARY EMBOLISM
QUADRA-PE study will evaluate the safety and effectiveness of the Katana™ Thrombectomy System LOS GATOS, Calif., Nov. 4, 2024 /PRNewswire/ — Akura Medical, a Shifamed portfolio company focused on reshaping the landscape of venous thromboembolism (VTE) care, announced today the US Food…
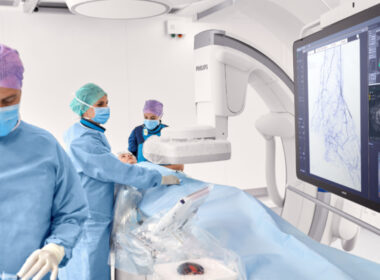
Philips enrolls first patient in U.S. clinical trial for innovative integrated single-device to treat peripheral artery disease
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced enrollment of the first patient in the U.S. THOR IDE clinical trial, which will study an innovative combined laser atherectomy and intravascular lithotripsy catheter developed by Philips, that integrates two critical PAD treatments into a single device. Procedures that previously required the use of two different devices can now be performed in a single procedure using a single device, simplifying workflows and procedures and potentially reducing the risk and improving outcomes for patients who might otherwise face multiple complex interventions.