DURHAM, N.C., Oct. 08, 2024 (GLOBE NEWSWIRE) — Humacyte, Inc. (Nasdaq: HUMA), a clinical-stage biotechnology platform company developing universally implantable, bioengineered human tissue at commercial scale, announced that its late-breaking abstract on the V007 Phase 3 clinical trial of the acellular tissue engineered vessel (ATEV™) in arteriovenous access for patients with end-stage renal disease was accepted for an oral presentation at the American Society of Nephrology’s (ASN) Kidney Week 2024. The late-breaking abstract titled “Prospective Randomized Trial of Humacyte’s Acellular Tissue Engineered Vessel Versus Autologous Arteriovenous Fistula for Hemodialysis Access” will be presented at the ASN meeting in San Diego on October 26, 2024.
Peripheral/Endo
Surmodics Receives FDA 510(k) Clearance for Pounce™ XL Thrombectomy System, Expanding the Pounce Thrombectomy Platform to Larger Peripheral Arteries up to 10 mm in Diameter
EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–Surmodics, Inc. (Nasdaq: SRDX), a leading provider of medical device and in vitro diagnostic technologies, today announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Pounce™ XL Thrombectomy System. “Securing FDA clearance for the Pounce XL Thrombectomy System is a major step […]
Humacyte to Host Virtual KOL Event to Discuss Case Studies on the Use of Acellular Tissue Engineered Vessel (ATEV™) in Vascular Trauma Treatment on September 30, 2024
DURHAM, N.C., Sept. 26, 2024 (GLOBE NEWSWIRE) — Humacyte, Inc. (Nasdaq: HUMA), a clinical-stage biotechnology platform company developing universally implantable, bioengineered human tissue at commercial scale, today announced it will host a virtual KOL event on Monday, September 30, 2024 at 8:00 AM ET. To register, click here.
AngioDynamics Initiates RECOVER-AV Clinical Trial Assessing AlphaVac F18⁸⁵ System in Treatment of Pulmonary Embolism and Long-Term Functional Outcomes
LATHAM, N.Y.–(BUSINESS WIRE)–AngioDynamics, Inc. (NASDAQ: ANGO), a leading and transformative medical technology company focused on restoring healthy blood flow in the body’s vascular system, expanding cancer treatment options and improving patient quality of life, today announced the launch of the Prospective, Multicenter, Multi-national, Single Arm Trial Evaluating the Efficacy, Safety […]
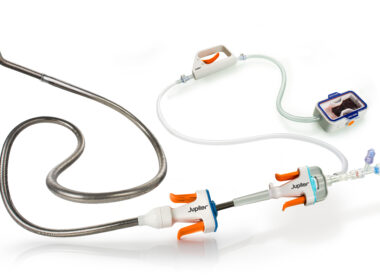
Jupiter Endovascular Announces First Patients Successfully Treated in First Clinical Study of Vertex Pulmonary Embolectomy System Using Endoportal Control
MENLO PARK, Calif.–(BUSINESS WIRE)–Jupiter Endovascular, Inc., a medical technology startup developing a new class of endovascular procedures using Endoportal Control™, announced today that the first two patients have been treated in SPIRARE I (NCT06571760), a multicenter study of the Vertex Pulmonary Embolectomy System using the company’s Endoportal Control™ platform technology. […]
CorMedix Inc. Announces New Commercial Agreement
BERKELEY HEIGHTS, N.J., Sept. 19, 2024 (GLOBE NEWSWIRE) — CorMedix Inc. (Nasdaq: CRMD), a biopharmaceutical company focused on developing therapeutic products for life-threatening diseases and conditions, today announced that it has entered into a new commercial supply contract with a top-tier mid-sized dialysis operator for the supply of DefenCath® (taurolidine and heparin) to dialysis clinics in the US. This new agreement will provide access to DefenCath for adult patients with kidney failure receiving hemodialysis through a central venous catheter at more than 250 outpatient dialysis clinics located across the United States.
Market Expansion for XARELTO in Peripheral Artery Disease Treatment through 2032: Forward-looking Insights and Sales Forecasts, Dynamics and Emerging Therapies – ResearchAndMarkets.com
DUBLIN–(BUSINESS WIRE)–The “XARELTO Market Size, Forecast, and Market Insight – 2032” report has been added to ResearchAndMarkets.com’s offering. “XARELTO Market Size, Forecast, and Market Insight – 2032” Post this The latest industry report provides a thorough analysis of the potential growth and impact of XARELTO for the treatment of Peripheral Artery Disease (PAD) in […]
ZONTIVITY Emerges as a Key Player in the Treatment of Peripheral Artery Disease with Promising Market Forecast Through 2032 – ResearchAndMarkets.com
September 17, 2024 12:09 PM Eastern Daylight Time DUBLIN–(BUSINESS WIRE)–The “ZONTIVITY Market Size, Forecast, and Market Insight – 2032” report has been added to ResearchAndMarkets.com’s offering. “ZONTIVITY Market Size, Forecast, and Market Insight – 2032” Post this ZONTIVITY, known for its efficacy in reducing thrombotic cardiovascular events, emerges as a significant pharmaceutical agent in […]
Philips announces FDA approval for enhanced LumiGuide guidewire and marks the 1000th patient treated with its breakthrough 3D device guidance technology
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the introduction of the 160cm FDA approved version of its unique LumiGuide endovascular navigation wire. This enhanced LumiGuide guidewire, which utilizes the company’s breakthrough Fiber Optic RealShape (FORS) technology, was used for the first time on a patient by Professor and vascular surgeon Carlos Timaran, MD, an internationally recognized expert in advanced endovascular techniques. He used the technology during a complex aortic aneurism repair operation, marking the 1000th patient treated milestone using FORS technology since first clinical use of the technology in 2020. The introduction of a longer FORS-enabled LumiGuide Navigation Wire enables US clinicians to visualize a broader range of catheters and expands usage of this technology to patients in America treated in centers equipped with LumiGuide.
Microbot Medical Announces the Successful Enrollment of 50% of the Patients in its Pivotal Human Clinical Trial for the LIBERTY Endovascular Robotic Surgical System
Completion of enrollment expected in Q4 with FDA submission for commercialization anticipated by the end of 2024 Completion of enrollment expected in Q4 with FDA submission for commercialization anticipated by the end of 2024