May 13, 2024 08:00 AM Eastern Daylight Time IRVINE, Calif.–(BUSINESS WIRE)–Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, has announced the online publication of the one-year results of the DETOUR2 Trial in the Journal of Vascular Surgery […]
Peripheral/Endo
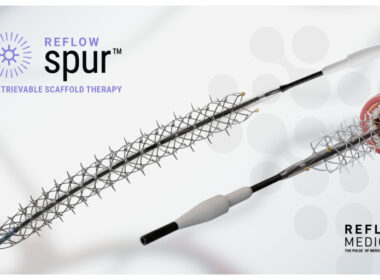
Reflow Medical Completes Enrollment in the DEEPER REVEAL Clinical Trial
May 09, 2024 07:00 AM Eastern Daylight Time SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical, Inc., a developer of innovative devices focused on cardiovascular disease, announces completion of enrollment in the DEEPER REVEAL clinical trial (NCT05358353) to evaluate the Reflow Spur™ Stent. Designated a Breakthrough Device by the FDA, the Spur is a […]
AngioDynamics’ APEX-AV Trial Results Assessing AlphaVac F18⁸⁵ System in Treatment of Pulmonary Embolism Presented at SCAI 2024 Scientific Sessions
LATHAM, N.Y.–(BUSINESS WIRE)–AngioDynamics, Inc. (NASDAQ: ANGO), a leading and transformative medical technology company focused on restoring healthy blood flow in the body’s vascular system, expanding cancer treatment options and improving patient quality of life, today announced that results from the Acute Pulmonary Embolism Extraction Trial with the AlphaVac System (APEX-AV) were […]
Endovascular Engineering Presents Positive Results from ENGULF Feasibility Trial with its Hēlo™ PE Thrombectomy System for the Treatment of Pulmonary Embolism
MENLO PARK, Calif., May 6, 2024 /PRNewswire/ — Endovascular Engineering, Inc. (“E2”), a pioneering medical device company at the forefront of advancing clot removal technologies for venous thromboembolism (VTE), proudly announced positive safety and feasibility results from its ENGULF US…
Provisio Medical Announces Physician Advisory Board
Experts in Vascular Surgery, Interventional Radiology and Interventional Cardiology to Help Guide Commercialization SAN DIEGO, May 6, 2024 /PRNewswire/ — Provisio Medical, Inc. today announced the formation of a Physician Advisory Board (PAB) comprising a multidisciplinary group of…
SonoVascular Enters into Strategic Collaboration with Lantheus Holdings, Inc. for Use of Microbubbles in Combination with SonoThrombectomy™ System for Treatment of Venous Thromboembolism
CHAPEL HILL, N.C., May 2, 2024 /PRNewswire/ — SonoVascular, Inc., a private medical device company focused on the development of its SonoThrombectomy™ System, a novel pharmaco-mechanical ultrasound facilitated thrombectomy system that utilizes microbubble-mediated cavitation as a core…
Penumbra Launches Lightning Flash 2.0 – Latest CAVT Technology Designed to Rapidly Remove Blood Clots
Lightning Flash™ 2.0 features advanced computer assisted vacuum thrombectomy (CAVT) software, designed for increased efficiency and sensitivity to enhance removal of venous thrombus and treatment of pulmonary emboli (PE) Combined with streamlined audio-visual feedback, Lightning Flash 2.0…
Bolt Medical Announces Completion of Enrollment of RESTORE ATK Pivotal Trial for the Unique Bolt Intravascular Lithotripsy System
Bolt Medical completes the enrollment of RESTORE ATK pivotal trial for the treatment of peripheral arterial disease with its unique intravascular lithotripsy system. CARLSBAD, Calif., April 22, 2024 /PRNewswire-PRWeb/ — Bolt Medical, Inc., a clinical stage medical device company is…
Innovative Aspiration Thrombectomy System by Expanse ICE Receives FDA Clearance for vessels of the peripheral arterial and venous systems
PLEASANTON, Calif., April 22, 2024 /PRNewswire/ — Expanse ICE announced today the ICE Aspiration System has received 510(k) clearance from the U.S. Food and Drug Administration. This announcement introduces a new and exciting player in the peripheral thrombectomy market. The ICE…
Viz.ai Wins 2024 Edison Award™ for Innovation for Second Year in a Row
April 19, 2024 09:51 AM Eastern Daylight Time SAN FRANCISCO–(BUSINESS WIRE)–Viz.ai, the leader in AI-powered disease detection and intelligent care coordination, today announced that it has been named an Edison Award Winner in the 2024 Edison Awards, for the second year in a row. Viz PE with right ventricle/left ventricle […]