BOSTON–(BUSINESS WIRE)–AVS (Amplitude Vascular Systems, Inc), an early-stage medical device company focused on safely and effectively treating severely calcified arterial disease with its PIVL™ therapy, announced the appointment of Elizabeth Galle as the company’s new Vice President of Clinical Affairs. She comes to AVS with more than two decades of leadership […]
Peripheral/Endo
Endospan Releases Early TRIOMPHE IDE Study Results at Society of Thoracic Surgeons
HERZLIA, Israel–(BUSINESS WIRE)–Endospan is a pioneer in off-the-shelf endovascular repair of aortic arch disease. As a notice of availability to educate potential referring physicians about the TRIOMPHE study, Endospan shared the 30-day results of the first 22 patients enrolled in the TRIOMPHE IDE study in a Late-Breaking presentation at the 60th […]
GORE RECEIVES FDA APPROVAL FOR BREAKTHROUGH ENDOVASCULAR DEVICE IN COMPLEX AORTIC ANEURYSMS
The GORE® EXCLUDER® Thoracoabdominal Branch Endoprosthesis approval brings the first branched, all-in-one solution for patients with thoracoabdominal aortic aneurysms and high-surgical risk patients with pararenal aortic aneurysms in patients with appropriate anatomy. “TAMBE is an innovative first step forward that enables us to provide care that’s both effective and timely for […]
“Sean Lyden MD, Professor and Chairman Vascular Surgery Cleveland Clinic on the mainstage at ISET 2024”
WEST CHESTER, Pa., Jan. 26, 2024 /PRNewswire/ — VESTECK, Inc. is excited to announce that the “SUTURE-TIGHT”tm catheter technology was presented at the iconic ISET meeting in Miami FL on January 24, 2024. During the main stage sessions, Sean Lyden MD, Professor and Chairman Vascular…
AngioDynamics Announces FDA 510(k) Clearance of Auryon XL Radial Access Catheter to Treat Peripheral Arterial Disease
LATHAM, N.Y.–(BUSINESS WIRE)–AngioDynamics, Inc. (NASDAQ: ANGO), a leading and transformative medical technology company focused on restoring healthy blood flow in the body’s vascular system, expanding cancer treatment options and improving patient quality of life, today announced that the United States Food and Drug Administration (FDA) has cleared the Auryon XL Catheter, […]
Nectero Medical Announces Initiation of Phase II/III Clinical Trial of the Nectero EAST® System for Treatment of Small- to Medium-Sized Abdominal Aortic Aneurysms
TEMPE, Ariz.–(BUSINESS WIRE)– #AbdominalAorticAneurysm–Nectero Medical, a clinical-stage biotechnology company pioneering novel therapies to treat aneurysmal disease and improve patients’ lives, today announced initiation of a Phase II/III clinical trial (stAAAble) to investigate the safety and efficacy of the Nectero Endovascular Aneurysm Stabilization Treatment (Nectero EAST®) System in patients with infrarenal abdominal aortic aneurysms (AAAs), maximum diameter 3.5 – 5.0cm. The Nectero EAST System is a single-use,
First U.S. Implantation of VasQ™ Since FDA De Novo was Granted
SPARTANBURG, S.C., Jan. 16, 2024 /PRNewswire/ — The first U.S. implantation of VasQ™ since FDA De Novo was granted was successfully performed by Dr. Ari Kramer, Director and principal surgeon of Vascular Access Surgery at Spartanburg Regional Hospital in Spartanburg, SC. Developed by…
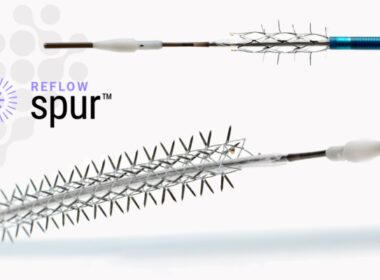
Reflow Medical Receives CE Mark for Bare Temporary Spur Stent System for Treating de novo or Restenotic Below-the-Knee (BTK) Lesions
San Clemente, CA—Jan. 15, 2024 – Reflow Medical, Inc., a developer of innovative medical devices focused on cardiovascular disease, announces it has received CE (Conformité Européenne) Mark certification in the European Union for the Bare Temporary Spur Stent System. The device is intended to treat de novo or restenotic lesions […]
Multidisciplinary panel of experts from prominent cardiovascular societies advocate for broader adoption of IVUS in peripheral interventions to improve patient care
Amsterdam, the Netherlands – Royal Philips, a global leader in health technology, today announced that a group of multidisciplinary cardiovascular specialists from prominent medical societies have issued a joint expert opinion on the advantages of intravascular ultrasound (IVUS) use in peripheral arterial and deep venous interventions compared with using angiography alone. […]
Endovascular Engineering’s Hēlo™ Thrombectomy System Receives IDE Approval to Start ENGULF Pivotal Trial for the Treatment of Pulmonary Embolism
MENLO PARK, Calif., Jan. 11, 2024 /PRNewswire/ — Endovascular Engineering (“E2”), a medical device company focused on the development and deployment of groundbreaking clot removal technologies that target venous thromboembolism (VTE), announced today FDA Investigational Device Exemption…